Cyclic Peptides in Drug Development: Unleashing Potential in Modern Medicine
Abstract
Cyclic peptides have captured significant interest in the field of drug development due to their unique structural properties and robust therapeutic potential. These molecules, characterized by a closed-loop structure formed through a covalent bond that connects the ends of the peptide chain, offer distinct advantages over linear peptides, including greater stability, enhanced specificity, and higher biological activity.
Introduction to Cyclic Peptides
Cyclic peptides are a class of naturally occurring molecules that play crucial roles in the biological defense mechanisms of various organisms. Found in a wide array of species, from plants and bacteria to animals, these compounds are involved in processes ranging from signaling to protection against predators and microbial invasion. Their notable presence in traditional medicinal practices across different cultures highlights their importance and diverse biological activities.
The defining feature of cyclic peptides is their ring-shaped structure, formed by a covalent bond linking the ends of the peptide chain. This configuration imparts significant stability and rigidity compared to their linear counterparts. The cyclic nature of these peptides not only enhances their resistance to proteolytic enzymes, which often degrade linear peptides quickly in biological environments, but also promotes a high degree of binding specificity and efficacy. As a result, cyclic peptides can engage more precisely with target molecules, making them highly effective in therapeutic applications.
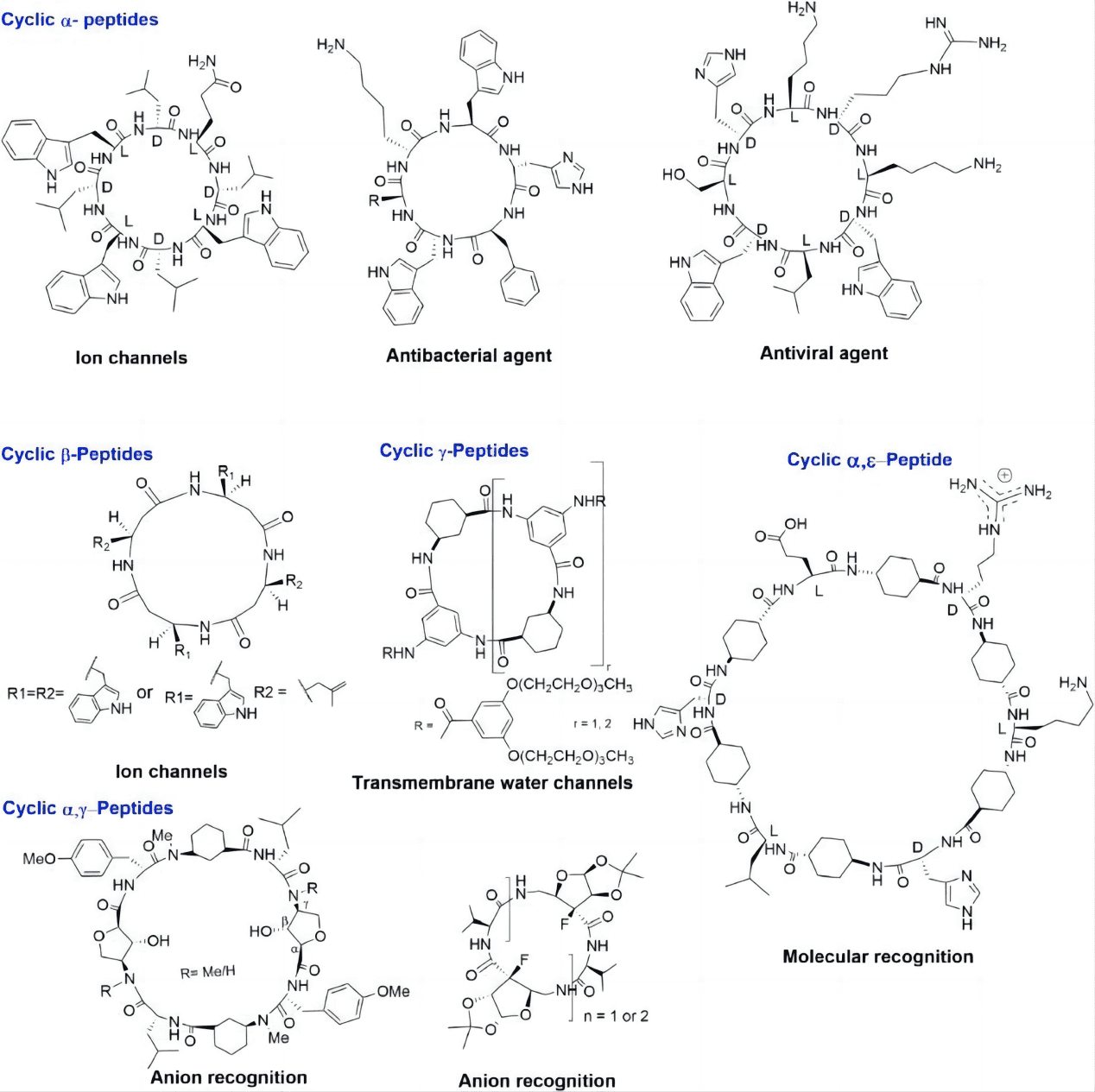
The advancement of scientific research has greatly expanded our understanding of cyclic peptides, revealing their potential to innovate modern therapeutic strategies. Their ability to mimic or block biological molecules, coupled with their inherent stability, makes them attractive candidates for drug development. These peptides offer promising avenues for targeting complex diseases, including those where traditional small molecule drugs and larger biologics may fall short.
As research continues, the role of cyclic peptides in drug development is increasingly recognized, prompting further exploration into their pharmacological potential. This growing interest is fueled by advancements in peptide synthesis and drug delivery technologies, which enhance the feasibility and effectiveness of cyclic peptides as therapeutic agents. Thus, cyclic peptides represent a potent and versatile tool in the development of next-generation treatments, bridging traditional natural compounds with cutting-edge medical innovation.
Structural Attributes and Advantages
The cyclic nature of these peptides contributes significantly to their stability against enzymatic degradation, which is a common limitation for linear peptides. This structural stability ensures that cyclic peptides retain their functional integrity longer in the physiological environment, making them ideal candidates for therapeutic use. Furthermore, the constrained conformation of cyclic peptides allows for a high degree of specificity in targeting. They can effectively bind to and modulate the activity of specific proteins involved in disease pathways, reducing the likelihood of off-target effects that often lead to adverse drug reactions.
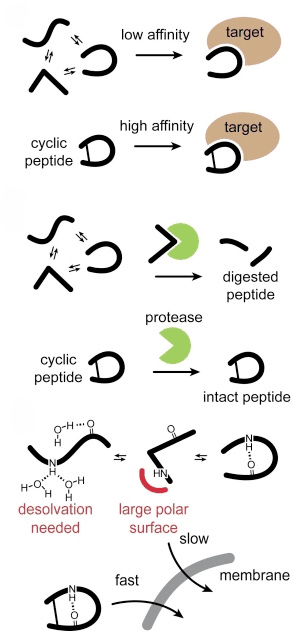
Effects of cyclization on binding affinity, stability, and membrane permeability
Technological Advances in Peptide Synthesis
The synthesis of cyclic peptides has evolved from rudimentary techniques to advanced methodologies that allow for precise assembly and modifications. Innovations such as solid-phase peptide synthesis (SPPS) and automated synthesizers have revolutionized the way cyclic peptides are produced. SPPS, in particular, facilitates the sequential addition of amino acids to a peptide chain anchored to a solid resin, making the synthesis process both efficient and scalable.
Moreover, the advent of technologies that allow for the incorporation of non-natural amino acids has expanded the toolkit available to drug developers. These synthetic amino acids can introduce properties such as increased stability to metabolic breakdown, enhanced binding affinity, or specific interactions with the biological target, which are not possible with natural amino acids alone.
High-Throughput Screening and Drug Discovery
High-throughput screening (HTS) technologies have been instrumental in accelerating the discovery and development of cyclic peptide drugs. Techniques like phage display and mRNA display utilize vast libraries of peptides displayed on the surface of viruses or linked to mRNA, respectively. These methods enable the rapid screening of millions of peptides to identify those with the highest affinity for a target molecule. This approach significantly reduces the time and cost associated with traditional drug discovery processes.
Phage display involves engineering bacteriophages to present peptides on their surface, which can then be screened against various targets to identify binders with high specificity and affinity. mRNA display offers similar benefits but has the added advantage of linking the phenotype of the peptide directly to its encoding mRNA, facilitating the identification and synthesis of successful candidates.
Applications in Targeting Intracellular and Extracellular Proteins
Cyclic peptides are uniquely effective in targeting both extracellular and intracellular proteins, a property that is highly valued in the design of drugs for a variety of diseases. Traditionally, cyclic peptide drugs have targeted extracellular molecules due to the challenges associated with membrane permeability. These drugs, often administered via injection, are used to neutralize or modulate proteins that are accessible in the circulatory system or located on cell surfaces.
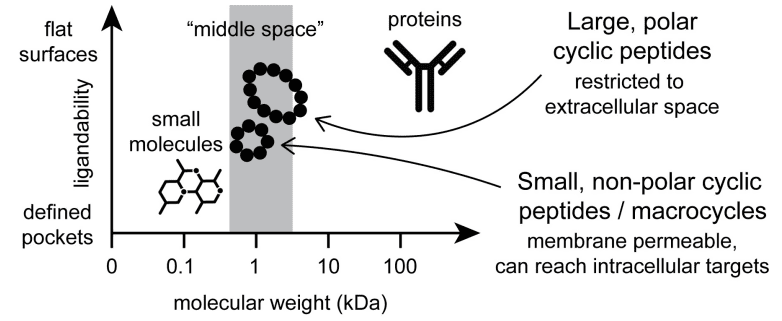
Comparison of cyclic peptide therapeutics to classical small molecules and biologics
The pursuit of cyclic peptides capable of intracellular targeting has intensified, driven by the need to tackle diseases that involve proteins within cells, such as cancer and genetic disorders. Recent advances have led to the development of cyclic peptides that can penetrate cell membranes and deliver their therapeutic effects directly to intracellular targets. This has been achieved through modifications that enhance their ability to cross lipid bilayers or by coupling them with cell-penetrating peptides that facilitate their entry into cells.
Challenges in Clinical Development
Despite their promising features, cyclic peptides face several challenges in clinical development, particularly concerning their pharmacokinetic properties. Issues such as poor oral bioavailability, rapid renal clearance, and susceptibility to proteolytic degradation can limit their therapeutic utility. To overcome these obstacles, researchers are focusing on innovative delivery systems, such as nanoparticle encapsulation, which can protect peptides from degradation and improve their absorption and distribution within the body.
Additionally, the development of cyclic peptides that can be orally administered remains a significant challenge. Strategies to enhance oral bioavailability include the design of peptides that are more resistant to enzymatic breakdown in the gastrointestinal tract and modifications that increase their permeability across the intestinal epithelium.
Future Directions and Innovations
Looking forward, the field of cyclic peptides is poised for transformative growth, driven by continuous advancements in synthesis technologies, screening methods, and computational drug design. The integration of artificial intelligence and machine learning into the cyclic peptide design process promises to revolutionize how these molecules are developed, enabling the rapid prediction and optimization of peptide structures with desired therapeutic properties.
Furthermore, the expanding understanding of the molecular mechanisms of diseases provides a growing number of targets for cyclic peptide drugs. As researchers continue to unravel the complex interactions within cells, cyclic peptides will likely play an increasingly important role in addressing previously untreatable conditions.
Conclusion
Cyclic peptides stand on the cutting edge of pharmaceutical innovation, showcasing a transformative potential in the field of drug development. With their ring-shaped molecular structure, these compounds provide a robust platform for addressing a myriad of therapeutic challenges. The intrinsic stability afforded by their cyclic nature, coupled with their high specificity and binding affinity, makes them exceptionally effective against targeted disease pathways. These properties minimize undesirable off-target effects and enhance drug efficacy, aligning perfectly with the goals of precision medicine.
The advances in synthetic techniques and high-throughput screening technologies have propelled cyclic peptides into the forefront of biomedical research. These technological strides have not only streamlined the production and discovery of cyclic peptides but have also broadened the scope of their application. Today, cyclic peptides are being engineered to target both extracellular and intracellular proteins, expanding their use from traditional areas such as antimicrobials and hormones to more complex diseases like cancer and genetic disorders. Moreover, the integration of non-natural amino acids and innovative delivery systems continues to overcome previous limitations, enhancing their pharmacokinetic profiles and therapeutic indices.
Looking to the future, the continued fusion of computational drug design with empirical science promises to further accelerate the pace of cyclic peptide development. Artificial intelligence and machine learning are poised to refine the predictive modeling of peptide interactions, reducing the time and cost associated with drug discovery and development. As the scientific community gains deeper insights into disease mechanisms at a molecular level, cyclic peptides are expected to play an increasingly vital role in developing next-generation therapeutics.
In conclusion, cyclic peptides embody a dynamic and promising class of bioactive compounds with the potential to revolutionize treatment paradigms across a spectrum of diseases, heralding a new era of drug development characterized by targeted, effective, and personalized therapeutic options.