Cimicifugae Rhizoma (Sheng Ma): Ancient Herb, Modern Science, and the Future of Herbal Medicine
Abstract
Cimicifugae Rhizoma (Sheng Ma), a staple in traditional Chinese medicine (TCM), has been used for centuries to treat ailments ranging from fever and inflammation to prolapse and menopausal discomfort. Recent scientific investigations have unveiled a rich chemical profile—particularly triterpenoid saponins and phenylpropanoids—underlying its wide-ranging pharmacological effects, including anti-inflammatory, antitumor, antioxidant, and antiviral activities. This blog post explores the herb’s traditional roots, modern clinical applications, and the growing use of DNA barcoding and chromatographic techniques for quality control. It also discusses future directions such as toxicity profiling, standardized processing, and emerging potential in cosmeceuticals and nutraceuticals. By bridging tradition with science, Cimicifugae Rhizoma is poised for a broader role in global integrative medicine.
Introduction – The Legacy of Cimicifugae Rhizoma
Cimicifugae Rhizoma, known as Sheng Ma in Traditional Chinese Medicine (TCM), is a time-honored medicinal herb with over 2,000 years of documented therapeutic use. It refers to the dried rhizomes of Cimicifuga foetida, Cimicifuga dahurica, and Cimicifuga heracleifolia, and it has been classified as a “top grade” herb since its first appearance in the Shennong’s Classic of Materia Medica.
Traditionally, Sheng Ma has been used to expel wind-heat, release skin eruptions, relieve sore throat and mouth ulcers, and address more systemic concerns like uterine prolapse, rectal prolapse, and postpartum bleeding. It was also noted in several ancient texts for its unique “Yang-Invigorating” property—making it a valuable herb for raising Qi and treating symptoms like chronic fatigue, diarrhea, and dizziness.
In addition to its medicinal use, Cimicifugae Rhizoma held dietary value in Chinese folk practices. Its tender shoots were often consumed as cold dishes or stir-fried, prized for their heat-clearing effects on the digestive tract and their ability to relieve inflammation-related ailments like toothache and throat swelling.
Today, over 80 proprietary Chinese medicinal formulations contain Cimicifugae Rhizoma as a core or supporting ingredient. Some widely used examples include Shengma Biejia Pills, Shengti Capsules, and Qingwei Powder, each designed to harness its anti-inflammatory, detoxifying, and guiding (channel ushering) effects. Notably, the herb’s ability to guide other medicines to the upper body has made it a strategic component in compound prescriptions.
Given the convergence of ancient documentation and modern clinical utility, Cimicifugae Rhizoma remains an enduring focus of pharmacological interest and an important link between traditional healing and modern science.
Inside the Chemistry – What Makes Sheng Ma Work?
The therapeutic versatility of Cimicifugae Rhizoma (Sheng Ma) stems from its rich and complex phytochemical profile. Modern phytochemical investigations have identified 348 distinct compounds, which collectively contribute to its wide-ranging pharmacological effects. Among these, triterpenoid saponins stand out as the most abundant and biologically potent group.
Over 200 triterpenoid saponins have been isolated, including major representatives like actein, cimigenol, and 23-epi-26-deoxyactein. These compounds are recognized for their anti-inflammatory, antitumor, and neuroprotective activities. Structurally, they can be grouped into several skeleton types—such as cimigenol-type, actein-type, and cimilactone-type—which influence their specific biological pathways.
In addition to saponins, phenylpropanoids form another key category, with ferulic acid (FA), isoferulic acid (IFA), and caffeic acid being particularly noteworthy. These compounds exhibit antioxidant, antiviral, and anti-aging properties, primarily through their ability to scavenge free radicals and modulate inflammatory signaling pathways.
The herb also contains chromones, such as cimifugin and norcimifugin, which display notable anti-inflammatory and analgesic effects. These are believed to play a role in alleviating conditions like sore throat, asthma, and dermatitis. Additional components include alkaloids, flavonoids, lignans, sterols, and volatile oils, each adding nuanced therapeutic potential.
Interestingly, the processing methods of Sheng Ma—such as honey stir-frying, vinegar processing, or charcoal stir-frying—can alter its chemical composition, enhancing desired effects or reducing potential toxicity. For instance, honey-processed Sheng Ma is preferred for strengthening the spleen and elevating Qi, while raw Sheng Ma is more commonly used for detoxification and treating fever.
Overall, the sophisticated chemistry of Cimicifugae Rhizoma underpins its ability to interact with multiple biological targets, aligning with the TCM principle of holistic healing. This chemical diversity continues to fuel both pharmacological research and the modernization of traditional herbal practices.
Proven Pharmacological Benefits – From Inflammation to Cancer
Cimicifugae Rhizoma (Sheng Ma) has gained considerable attention in recent years for its scientifically validated pharmacological activities, which span multiple therapeutic domains. Modern research has confirmed that the herb’s diverse chemical constituents—particularly triterpenoid saponins and phenylpropanoids—contribute to its broad-spectrum biological effects.
One of the most well-documented actions of Cimicifugae Rhizoma is its anti-inflammatory capability. Studies have shown that its active compounds inhibit pro-inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), and nitric oxide (NO). These effects are mediated by pathways like NF-κB and MAPK, making the herb valuable in treating inflammation-related disorders such as asthma, dermatitis, and colitis.
Another major area of interest is its antitumor potential. Compounds like actein and cimigenol have been found to induce apoptosis, cell cycle arrest, and autophagy in cancer cell lines. These saponins demonstrate efficacy against breast, lung, liver, and colon cancers by disrupting mitochondrial function and suppressing signaling pathways like PI3K/Akt and JAK/STAT.
Cimicifugae Rhizoma also exhibits robust antioxidant activity, largely attributed to ferulic acid (FA) and isoferulic acid (IFA). These compounds scavenge reactive oxygen species (ROS), protect cellular structures, and enhance endogenous antioxidant enzymes—critical in mitigating oxidative stress linked to aging, neurodegeneration, and chronic disease.
The herb’s antiviral effects are equally promising. Extracts and isolated phenolic acids have shown inhibitory activity against hepatitis B virus (HBV), respiratory syncytial virus (RSV), influenza, and coronaviruses. These effects are believed to result from a combination of direct antiviral action and immune modulation.
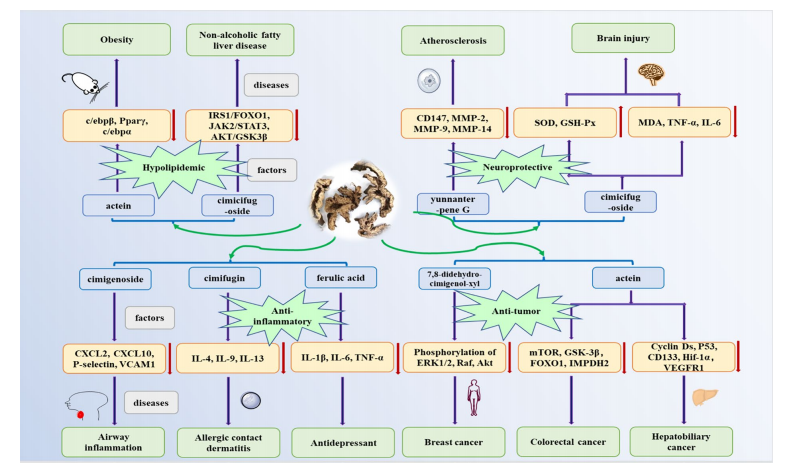
Fig. 1 Changes in biological indicators of Cimicifugae Rhizoma on anti-infammatory, anti-tumor, hypolipidemic and neuroprotective activities
Additionally, Cimicifugae Rhizoma has demonstrated benefits in menopause symptom relief, neuroprotection, and cardiovascular support, marking it as a truly multi-functional botanical medicine.
Clinical Uses and Quality Control – Tradition Meets Technology
While the historical value of Cimicifugae Rhizoma (Sheng Ma) is well established in traditional Chinese medicine (TCM), its relevance in modern clinical applications continues to grow—supported by both empirical use and scientific standardization. Today, the herb is incorporated into therapies for a wide spectrum of conditions, ranging from autoimmune and infectious diseases to gynecological and gastrointestinal disorders.
Clinically, Sheng Ma has shown notable benefits in managing conditions such as chronic hepatitis B, systemic lupus erythematosus, herpes zoster, menopausal symptoms, and recurrent oral ulcers. In several randomized clinical studies, preparations containing Cimicifugae Rhizoma—such as Ximingting Tablets and Qingwei Powder—demonstrated efficacy in reducing symptom severity, improving immune response, and enhancing patient quality of life. When used alongside conventional treatments like adefovir dipivoxil or azithromycin, the herb often enhances therapeutic outcomes without significant adverse effects.
However, clinical effectiveness is highly dependent on species authenticity and chemical consistency—a challenge given the herb’s multi-origin nature (Cimicifuga foetida, C. dahurica, C. heracleifolia) and morphological similarity to adulterants. This has led to a growing emphasis on advanced quality control strategies to ensure efficacy and safety.
Modern approaches to quality assurance include:
DNA barcoding (e.g., ITS2 region analysis) for precise species identification
Chemical fingerprinting via HPLC, UPLC-MS, and GC-MS to detect bioactive markers like isoferulic acid, ferulic acid, and caffeic acid
Chemometric modeling to differentiate raw from processed materials and distinguish between authentic and counterfeit sources
The Chinese Pharmacopoeia currently mandates that Cimicifugae Rhizoma products contain at least 0.10% isoferulic acid, but researchers argue for multi-marker assessment to better reflect the herb’s full pharmacological profile.
In bridging traditional knowledge with technological precision, Cimicifugae Rhizoma exemplifies how herbal medicine can be modernized without losing its cultural and therapeutic roots.
Future Horizons – Toward Safe and Effective Global Use
As the global demand for plant-based therapies continues to rise, Cimicifugae Rhizoma (Sheng Ma) stands at a promising crossroads between traditional healing and evidence-based medicine. Despite centuries of documented use and emerging pharmacological validation, several critical research gaps remain that must be addressed for its safe, standardized, and widespread application.
One major challenge is the multi-target complexity inherent in herbal medicines. Cimicifugae Rhizoma contains hundreds of compounds that may act synergistically or antagonistically, depending on dosage, preparation method, and individual patient factors. To fully align with modern pharmacological expectations, multi-omics strategies—such as genomics, metabolomics, and network pharmacology—should be applied to unravel these compound interactions and elucidate mechanisms of action in a holistic framework.
Additionally, standardized processing protocols are urgently needed. Raw and processed forms of Sheng Ma differ markedly in their chemical compositions and therapeutic effects. The lack of universally accepted guidelines for processing methods like honey stir-frying or charcoal treatment contributes to variability in efficacy and safety. Establishing these protocols would greatly enhance reproducibility in both clinical trials and commercial production.
Another priority is comprehensive toxicity profiling, particularly regarding hepatotoxicity observed in high-dose or prolonged use scenarios. While clinical data suggest good tolerability in most applications, sex-based toxicity differences—notably higher sensitivity in females—must be systematically studied and factored into dosage recommendations.
Furthermore, the potential for new applications beyond traditional domains is gaining traction. Preliminary studies point to uses in cosmeceuticals, nutraceuticals, and anti-aging formulations, driven by the herb’s antioxidant, skin-lightening, and anti-inflammatory effects. With appropriate safety evaluation, Cimicifugae Rhizoma could become a valuable player in the wellness and personal care industries.
The future of Sheng Ma depends on bridging traditional wisdom with rigorous science. By doing so, this age-old botanical could become a globally trusted component in modern therapeutic and preventive healthcare systems.
References
Zhang, Q., Wei, W., Jin, X., Lu, J., Chen, S., Ogaji, O. D., … & Li, J. (2024). Traditional uses, phytochemistry, pharmacology, quality control and clinical studies of Cimicifugae Rhizoma: A comprehensive review. Chinese Medicine, 19(66).
https://doi.org/10.1186/s13020-024-00937-7
Li, P., & Liu, C. (2022). Origin, changes and analysis of efficacy of Cimicifugae Rhizoma. Chinese Journal of Experimental Traditional Medical Formulae, 28, 218–226.
https://doi.org/10.13422/j.cnki.syfjx.20220796
Chen, Q., & Wang, Z. (2019). Research progress in chemical constituents and pharmacological effects of Cimicifugae Rhizoma. Modern Chinese Medicine, 21(3), 363–367.
https://doi.org/10.13313/j.issn.1673-4890.20190328
Liu, Y., & Chen, X. (2021). Future directions in research on Chinese medicinal herbs: Integrating omics and toxicity evaluation. Journal of Ethnopharmacology, 275, 114098.
https://doi.org/10.1016/j.jep.2021.114098
Xie, Y., & Zhao, T. (2021). Anti-inflammatory and anti-cancer properties of Cimicifugae Rhizoma in molecular signaling. Chinese Journal of Natural Medicines, 19(10), 731–739.
https://doi.org/10.1016/S1875-5364(21)60091-6
Zhou, S., & Wang, Y. (2022). Progress in DNA barcoding technology for quality control of Chinese medicinal materials. Chinese Herbal Medicines, 14(3), 447–453.