CCR5 Inhibitors: Promising Therapeutics for HIV-1 and Beyond
Abstract
CCR5 is a crucial chemokine receptor found on immune cells that facilitates immune cell activation and inflammation-related disease progression. It also serves as a co-receptor for HIV-1 entry into host cells, making it an appealing target for HIV-1 treatment. CCR5 inhibitors have shown promise in treating HIV-1, and ongoing research into this protein could lead to innovative therapeutic approaches for other ailments. Thus, CCR5 is a critical protein with noteworthy consequences for immune function and viral infections.
Role of CCR5 in the immune system
CCR5 is a crucial protein receptor that plays a pivotal role in the immune system’s response to infections and inflammation. Pathogens such as bacteria or viruses activate an immune response, leading to the recruitment of immune cells to the site of infection. CCR5 is instrumental in the recruitment of specific immune cells, such as T cells and macrophages, to the site of infection, where they help to clear the pathogen. Moreover, CCR5 plays an important role in the migration of immune cells to lymphoid organs, where they can interact with other immune cells and mount a more effective immune response. A deeper understanding of CCR5’s function in immune regulation could lead to the discovery of novel therapies for various diseases.
CCR5 as a co-receptor for HIV entry:
To facilitate its entry into cells, HIV binds to the CD4 receptor on immune cells such as T cells, and CCR5 serves as a co-receptor. CCR5 plays a role not only in the immune system but also in HIV infection. CCR5 inhibitors have been developed as a treatment for HIV to prevent viral entry into cells by blocking the interaction between the virus and the receptor. Maraviroc is an FDA-approved CCR5 inhibitor used in HIV treatment.
CCR5 inhibitors as a treatment for HIV:
CCR5 inhibitors are effective in the treatment of HIV, particularly in patients with virus strains that use CCR5 as a co-receptor for entry. Clinical trials have demonstrated that Maraviroc, in combination with other antiretroviral drugs, can suppress HIV viral load and improve CD4 cell counts in treatment-naive and treatment-experienced patients. Maraviroc is generally well-tolerated, with common side effects including diarrhea, nausea, and headache. However, the use of CCR5 inhibitors can be limited by the emergence of drug-resistant strains of the virus, which can develop in patients who do not respond to treatment.
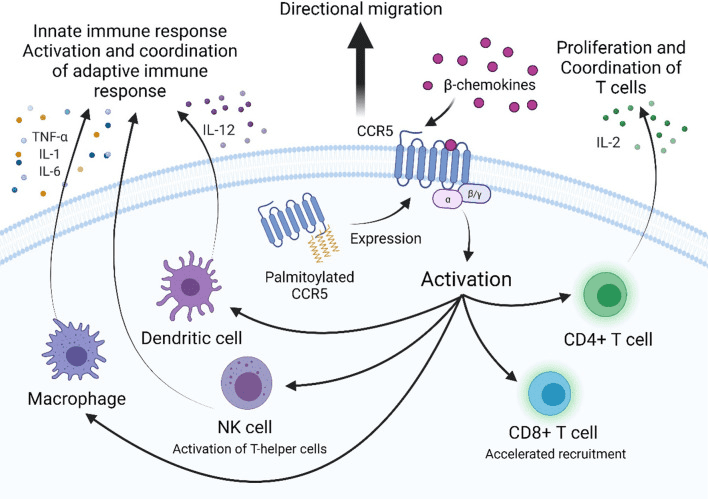
Figure 1[2] CCR5 is a G-protein coupled receptor that is involved in the activation and coordination of the innate and adaptive immune response.
The CCR5 inhibitors associated with the treatment of HIV are as follows :
- Maraviroc is an FDA-approved medication for treating HIV infection. It functions by inhibiting the CCR5 receptor, which HIV utilizes as a co-receptor to enter cells. By blocking the communication between the virus and the receptor, Maraviroc prevents viral entry and replication. It is usually used with other antiretroviral drugs and is successful in suppressing HIV viral load and enhancing CD4 cell counts in both treatment-naive and treatment-experienced patients. Common side effects of Maraviroc include diarrhea, nausea, and headache. Maraviroc is available in the form of an oral tablet.
- Vicriviroc, currently under investigation for HIV treatment, is a CCR5 inhibitor like Maraviroc, which blocks the CCR5 receptor to prevent HIV entry into cells. Although not yet approved by the FDA for HIV treatment, Vicriviroc has shown promising results in clinical trials and is generally well-tolerated with common side effects such as diarrhea, nausea, and headache. Nevertheless, more research is required to determine its safety and efficacy for HIV treatment.
- Cenicriviroc is a drug that functions as a dual CCR2/CCR5 inhibitor, and it has been researched for its potential to treat various inflammatory disorders, including HIV infection. By blocking the CCR2 and CCR5 receptors, Cenicriviroc reduces inflammation and restricts HIV from entering cells. Fatigue and diarrhea are common side effects of Cenicriviroc, according to clinical studies, and while it has shown promise as a possible HIV therapy, further research is needed to establish its safety and efficacy. The FDA has not yet authorized Cenicriviroc for HIV treatment.
- Aplaviroc is an investigational drug that belongs to the class of CCR5 inhibitors, similar to Maraviroc. It was being developed as a treatment for HIV but its development was halted due to safety concerns related to liver toxicity. It worked by blocking the CCR5 receptor, preventing HIV from entering cells. It has not been approved by the FDA for use in HIV treatment.
- TAK-779 is a CCR5 receptor antagonist that is being studied as a potential treatment for HIV. It works by blocking the CCR5 receptor that is used by the virus to enter and infect immune cells, thus potentially inhibiting HIV infection. While preclinical studies have shown promise, further clinical research is needed to determine the safety and effectiveness of TAK-779 before it can be used as a therapy for HIV.
- Vicriviroc maleate is an investigational medication being studied for the treatment of HIV infection. It is a CCR5 inhibitor that blocks the CCR5 receptor to prevent HIV from entering and infecting immune cells. Vicriviroc maleate is an orally administered salt form of Vicriviroc. It is still in clinical trials and has not yet been approved by the FDA for use in HIV treatment. Common side effects reported in clinical studies include diarrhea, nausea, and headache. Further research is needed to determine the safety and efficacy of Vicriviroc maleate for the treatment of HIV.
- TAK-220 is an oral CCR5 antagonist that selectively inhibits the binding of RANTES and MIP-1α to CCR5 with IC50 values of 3.5 nM and 1.4 nM, respectively. However, TAK-220 has no effect on CCR1, CCR2b, CCR3, CCR4, or CCR7. TAK-220 is also a selective inhibitor of HIV-1, with EC50 values ranging from 0.55nM to 1.7nM for various HIV-1 strains. The EC90 values for HIV-1 range from 4nM to 28nM. TAK-220 has shown potential as a therapeutic agent for HIV-1, but further research is necessary to evaluate its safety and efficacy.
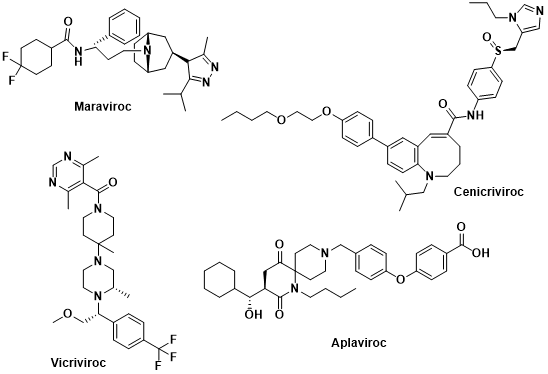
Figure 2 Structure of some inhibitors
Genetic mutations in CCR5:
Genetic mutations in CCR5 have been found to provide natural resistance to HIV infection. The most prominent of these mutations is the delta-32 mutation, which leads to the production of a non-functional truncated CCR5 protein. Individuals with two copies of the delta-32 mutation are completely resistant to HIV infection, while those with one copy may experience slower disease progression. Other CCR5 mutations have also been linked to modified susceptibility to HIV infection. These findings have sparked interest in developing gene therapies targeting CCR5 for HIV treatment.
Other diseases and conditions associated with CCR5:
Beyond its involvement in HIV, CCR5 has also been associated with other diseases and disorders. Research has indicated that CCR5 may contribute to the advancement and onset of certain cancers, including breast cancer and melanoma. Additionally, CCR5 has been linked to autoimmune conditions such as rheumatoid arthritis and multiple sclerosis, as well as cardiovascular disease. Nevertheless, the exact function of CCR5 in these disorders is not entirely clear and necessitates further investigation.
Conclusion and future directions:
To summarize, CCR5 is a significant protein receptor in the immune system that has implications for various diseases, including HIV, cancer, and autoimmune disorders. CCR5 inhibitors such as Maraviroc have shown promise in treating HIV, while genetic mutations in CCR5 provide natural resistance to HIV infection.
Future research on CCR5’s therapeutic potential may lead to the discovery of novel treatments for HIV and other diseases. This could involve developing new CCR5 inhibitors, gene therapies that target CCR5, or investigating CCR5’s role in cancer and autoimmune disorders to develop new therapies. Overall, understanding CCR5’s function and potential therapeutic applications will continue to be a vital area of research in the medical field.
Moreover, exploring the efficacy of CCR5 inhibitors in HIV treatment, in combination with other antiretroviral drugs, can be pursued to prevent the emergence of drug-resistant HIV strains. Investigating the potential long-term impacts of CCR5 inhibitors on HIV-positive individuals can also help determine the safety and effectiveness of these therapies. Ultimately, ongoing research on CCR5 has the potential to yield substantial advancements in treating a variety of illnesses and enhancing overall health outcomes.
References
- Zhang Z, Wang Q, Yao J, Zhou X, Zhao J, Zhang X, Dong J, Liao L. Chemokine Receptor 5, a Double-Edged Sword in Metabolic Syndrome and Cardiovascular Disease. Front Pharmacol. 2020 Mar 3;11:146. doi 10.3389/fphar.2020.00146. PMID: 32194402; PMCID: PMC7063056.
- Mohamed H, Gurrola T, Berman R, Collins M, Sariyer IK, Nonnemacher MR, Wigdahl B. Targeting CCR5 as a Component of an HIV-1 Therapeutic Strategy. Front Immunol. 2022 Jan 20;12:816515. doi: 10.3389/fimmu.2021.816515. PMID: 35126374; PMCID: PMC8811197.
- Fantuzzi L, Tagliamonte M, Gauzzi MC, Lopalco L. Dual CCR5/CCR2 targeting: opportunities for the cure of complex disorders. Cell Mol Life Sci. 2019 Dec;76(24):4869-4886. doi 10.1007/s00018-019-03255-6. Epub 2019 Aug 3. PMID: 31377844; PMCID: PMC6892368.
- Aldinucci D, Casagrande N. Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer. Int J Mol Sci. 2018 May 16;19(5):1477. doi: 10.3390/ijms19051477. PMID: 29772686; PMCID: PMC5983686.
- Hemmatazad H, Berger MD. CCR5 is a potential therapeutic target for cancer. Expert Opin Ther Targets. 2021 Apr;25(4):311-327. doi 10.1080/14728222.2021.1902505. Epub 2021 Jun 14. PMID: 33719836.
- Brelot A, Chakrabarti LA. CCR5 Revisited: How Mechanisms of HIV Entry Govern AIDS Pathogenesis. J Mol Biol. 2018 Aug 17;430(17):2557-2589. doi: 10.1016/j.jmb.2018.06.027. Epub 2018 Jun 19. PMID: 29932942.
- Necula D, Riviere-Cazaux C, Shen Y, Zhou M. Insight into the roles of CCR5 in learning and memory in normal and disordered states. Brain Behav Immun. 2021 Feb;92:1-9. doi 10.1016/j.bbi.2020.11.037. Epub 2020 Dec 1. PMID: 33276089.