Casein Kinase 1δ/ε: The Molecular Switch Linking Circadian Rhythms to Cancer, Metabolism, and Brain Health
Abstract
Casein Kinase 1 delta and epsilon (CK1δ/ε) have emerged as pivotal regulators of human biology, functioning as dynamic “molecular switches” that influence circadian rhythms, cancer progression, metabolic health, and neurological disorders. Their unique ability to alter substrate preference through autophosphorylation of the C-terminal tail allows CK1δ/ε to toggle between stabilizing and destabilizing protein pathways, most notably in the regulation of the circadian protein PER2. Beyond timekeeping, CK1δ/ε phosphorylation events drive oncogenic translation, shape adipogenesis, and modulate dopamine signaling, linking these kinases to diverse pathologies including sleep disorders, cancer, obesity, addiction, and Alzheimer’s disease. The therapeutic potential of CK1δ/ε lies in the development of isoform-selective and dual inhibitors capable of biasing the phosphoswitch toward protective outcomes. As research advances, CK1δ/ε stand out as central hubs for drug discovery, where insights from circadian biology converge with translational medicine.
The Kinase That Acts Like a Switch
Protein kinases are often portrayed as simple on–off regulators, adding phosphate groups to target proteins in a highly predictable fashion. Yet some kinases, such as Casein Kinase 1 delta and epsilon (CK1δ/ε), are far more versatile. These enzymes act more like molecular switches than binary toggles, dynamically changing their substrate preference depending on their own phosphorylation state. This ability allows them to play dual and sometimes opposing roles in cellular regulation.
CK1δ/ε are part of the Casein Kinase 1 family, which is widely expressed across tissues and involved in processes ranging from DNA repair to vesicle trafficking. What makes CK1δ/ε unique is their autophosphorylated C-terminal tail, which alters the configuration of the enzyme’s anion-binding pockets. These pockets normally guide CK1δ/ε to specific consensus motifs on substrate proteins. However, when the tail is phosphorylated, the binding landscape changes, steering the kinase toward alternative sites. In effect, CK1δ/ε can flip between different functional “modes.”
One of the best-characterized examples of this switch is found in the circadian clock protein PER2. CK1δ/ε can phosphorylate the FASP (Familial Advanced Sleep Phase) region of PER2, stabilizing the protein and lengthening circadian period. Under different tail-phosphorylation conditions, the kinase instead phosphorylates a degron motif, triggering PER2 degradation and shortening the circadian cycle. The same enzyme thus has two radically different outputs: one that protects PER2 and one that destroys it.
This phosphoswitch mechanism has profound implications. It provides a biochemical explanation for why mutations in CK1δ/ε or in PER2 can lead to circadian rhythm disorders such as Familial Advanced Sleep Phase Syndrome (FASPS). Moreover, it highlights how subtle structural changes in an enzyme can reshape entire physiological systems.
Beyond circadian biology, the CK1δ/ε phosphoswitch framework suggests a broader paradigm: kinases are not merely static signal relays but adaptive regulators that can rewire cellular circuits depending on context. This versatility makes CK1δ/ε both challenging and exciting as drug targets. By understanding how the switch operates, researchers hope to design therapies that bias CK1δ/ε activity toward protective pathways—whether in sleep regulation, cancer suppression, or neurodegeneration.
Resetting the Clock: Circadian Rhythm and PER2
At the heart of the body’s internal timing system lies the circadian clock—a finely tuned network of proteins that generate 24-hour rhythms in physiology and behavior. One of the central players in this mechanism is PER2 (Period 2), a protein whose stability and degradation set the pace of the circadian cycle. Casein Kinase 1 delta and epsilon (CK1δ/ε) are the key regulators of PER2, acting through a remarkable phosphoswitch that determines whether the protein is stabilized or destroyed.
The circadian system operates through transcription–translation feedback loops. Clock genes are transcribed and translated into proteins, which in turn inhibit their own expression, creating rhythmic oscillations. PER2 is a core repressor in this loop. The amount of PER2 present in the cell at any given time helps establish when the feedback cycle resets, thereby determining the length of the circadian period. This makes the regulation of PER2 by CK1δ/ε essential to maintaining a stable and accurate daily rhythm.
CK1δ/ε phosphorylate PER2 at two critical regions. In the FASP (Familial Advanced Sleep Phase) region, phosphorylation stabilizes PER2, extending its half-life and lengthening the circadian cycle. In contrast, phosphorylation of a degron motif marks PER2 for rapid degradation, shortening the cycle. The balance between these two outcomes—the FASP “protective” pathway and the degron “destructive” pathway—acts as a molecular switch that fine-tunes circadian timing.
Human genetics illustrates the importance of this balance. Mutations in PER2 that affect the FASP region, or mutations in CK1δ/ε that alter substrate preference, lead to Familial Advanced Sleep Phase Syndrome (FASPS), where individuals consistently fall asleep and wake much earlier than the typical population. Conversely, variants that destabilize PER2 more slowly are associated with Delayed Sleep Phase Disorder (DSPD), characterized by late-night wakefulness and difficulty rising in the morning.
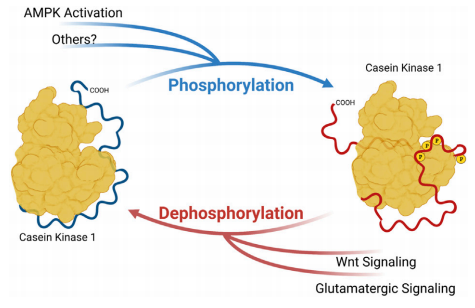
FIGURE 1 | Phosphorylation of Casein Kinase 1 Carboxyterminal tail influences substrate preference.
This precise regulation of PER2 exemplifies how small biochemical events can have dramatic effects on human behavior. By linking the molecular activity of CK1δ/ε to sleep-wake cycles, researchers have uncovered a clear pathway from enzyme activity to clinical phenotype. Understanding this mechanism not only provides insight into circadian biology but also highlights therapeutic opportunities: targeting CK1δ/ε to restore PER2 stability could help manage circadian rhythm disorders and improve overall sleep health.
Beyond Timekeeping: CK1δ/ε in Cancer, Metabolism, and the Brain
Although Casein Kinase 1 delta and epsilon (CK1δ/ε) are best known for their roles in circadian rhythm regulation, their influence extends well beyond the biological clock. These kinases serve as central regulators in pathways tied to cancer progression, metabolic control, and neurological function, demonstrating how circadian mechanisms are deeply woven into broader aspects of human health and disease.
In cancer biology, CK1δ/ε regulate proteins that influence tumor growth, cell communication, and metastasis. A prime example is Connexin-43, a gap junction protein that controls intercellular communication. Phosphorylation by CK1δ alters Connexin-43 function, weakening cell–cell adhesion and promoting migration—two hallmarks of metastatic cancer. Additionally, CK1δ phosphorylates 4EBP1, a translational repressor. When inactivated, 4EBP1 no longer blocks cap-dependent translation, enabling the synthesis of oncogenic proteins such as MYC and factors that drive adipogenesis like PPARγ. By deregulating both communication and translation, CK1δ/ε help create conditions that favor tumor proliferation and survival.
The kinases also play critical roles in metabolic regulation. Studies have shown that CK1ε interacts with AMPK, a central energy-sensing kinase, linking nutrient status to downstream metabolic control. Through 4EBP1 and PPARγ, CK1δ/ε influence fat cell differentiation, glucose metabolism, and insulin sensitivity. In experimental models, inhibition of CK1δ/ε has been shown to improve glucose tolerance and reduce adipose tissue mass, highlighting their potential as therapeutic targets for obesity and type 2 diabetes.
In the nervous system, CK1δ/ε modulate signaling pathways that affect learning, memory, and addiction biology. A notable example is DARPP-32, a phosphoprotein that integrates dopamine and glutamate signaling in neurons. Phosphorylation of DARPP-32 by CK1δ/ε shifts the balance between protein phosphatase 1 (PP1) and protein kinase A (PKA), influencing reward circuits and drug sensitivity. This mechanism has implications not only for substance use disorders but also for neurodegenerative diseases. In Alzheimer’s disease models, CK1δ/ε activity has been linked to tau hyperphosphorylation and amyloid pathology. Inhibition of these kinases improved cognition and reduced amyloid burden in preclinical studies, pointing to a possible therapeutic avenue for cognitive decline.
Together, these findings emphasize that CK1δ/ε function as multi-system regulators. What began as a story of circadian control has expanded into oncology, metabolism, and neuroscience, reinforcing the idea that manipulating CK1δ/ε could impact diverse disease landscapes.
Outlook: CK1δ/ε as a Hub for Drug Discovery
The discovery of CK1δ/ε’s phosphoswitch has shifted these enzymes from niche circadian regulators to promising drug discovery targets with broad therapeutic relevance. Their ability to act as adaptive molecular switches—stabilizing or destabilizing key proteins depending on context—makes them uniquely positioned at the crossroads of timekeeping, cancer, metabolism, and brain health.
A central challenge in drug development is selectivity. Since CK1δ and CK1ε share overlapping but distinct substrate preferences, designing inhibitors that can selectively bias one isoform over the other could allow for tailored therapeutic strategies. For example, targeting CK1δ may be more relevant in cancer, where its regulation of Connexin-43 and 4EBP1 promotes metastasis and MYC-driven translation. On the other hand, CK1ε’s role in AMPK cross-talk and neuronal signaling makes it attractive for metabolic and neurological disorders.
The potential of dual CK1δ/ε inhibition should not be overlooked. In preclinical models of Alzheimer’s disease, blocking both isoforms reduced amyloid burden and improved cognition, suggesting that broader inhibition may be beneficial when pathways are redundant or synergistic. Similarly, dual inhibitors such as SR-3029 have shown anticancer activity by suppressing MYC translation. However, careful calibration is needed to avoid disrupting circadian rhythms excessively, which could lead to side effects like sleep disturbances or metabolic imbalance.
Looking forward, precision medicine will likely play a decisive role. Genetic variants in PER2, CK1δ, and CK1ε already provide biomarkers for sleep disorders. Future therapies could use such biomarkers to stratify patients most likely to benefit from CK1δ/ε modulators, whether for circadian rhythm correction, metabolic disease management, or neuroprotection.
Another promising frontier is combination therapy. Because CK1δ/ε intersect with pathways like PI3K/AKT, AMPK, and dopamine signaling, co-targeting strategies may unlock synergistic effects. For example, umbralisib, a dual PI3Kδ/CK1ε inhibitor, illustrates how blending kinase targets can expand therapeutic impact.
In sum, CK1δ/ε represent a hub for drug discovery, uniting chronobiology with translational medicine. By “tuning the switch,” researchers can move beyond simply correcting circadian rhythm and harness CK1δ/ε inhibition to tackle cancer, diabetes, Alzheimer’s, and beyond. This integrative perspective positions CK1δ/ε not just as clock kinases, but as master regulators of human health.
References
Meng, Q. J., Maywood, E. S., Bechtold, D. A., Lu, W. Q., Li, J., Gibbs, J. E., … & Loudon, A. S. (2010). Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proceedings of the National Academy of Sciences, 107(34), 15240–15245.
https://doi.org/10.1073/pnas.1005101107
Partch, C. L., Green, C. B., & Takahashi, J. S. (2014). Molecular architecture of the mammalian circadian clock. Trends in Cell Biology, 24(2), 90–99.
https://doi.org/10.1016/j.tcb.2013.07.002
Philpott, J. M., Narasimamurthy, R., Ricci, C. G., Freeberg, A. M., Hunt, S. R., Yee, L. E., … & Partch, C. L. (2020). Casein kinase 1 dynamics underlie substrate selectivity and the PER2 circadian phosphoswitch. eLife, 9, e52343.
https://doi.org/10.7554/eLife.52343
Takahashi, J. S. (2017). Transcriptional architecture of the mammalian circadian clock. Nature Reviews Genetics, 18(3), 164–179.
https://doi.org/10.1038/nrg.2016.150
Toh, K. L., Jones, C. R., He, Y., Eide, E. J., Hinz, W. A., Virshup, D. M., … & Ptáček, L. J. (2001). An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science, 291(5506), 1040–1043.
https://doi.org/10.1126/science.1057499
Xu, Y., Padiath, Q. S., Shapiro, R. E., Jones, C. R., Wu, S. C., Saigoh, N., … & Ptáček, L. J. (2005). Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature, 434(7033), 640–644.
https://doi.org/10.1038/nature03453
Gallego, M., & Virshup, D. M. (2007). Post-translational modifications regulate the ticking of the circadian clock. Nature Reviews Molecular Cell Biology, 8(2), 139–148.
https://doi.org/10.1038/nrm2106
Zhou, M., Kim, J. K., Eng, G. W. L., Forger, D. B., & Virshup, D. M. (2015). A period2 phosphoswitch regulates and temperature compensates circadian period. Molecular Cell, 60(1), 77–88.
https://doi.org/10.1016/j.molcel.2015.08.022
Knippschild, U., Krüger, M., Richter, J., Xu, P., García-Reyes, B., Peifer, C., … & Bischof, J. (2014). The CK1 family: contribution to cellular stress response and its role in carcinogenesis. Frontiers in Oncology, 4, 96.
https://doi.org/10.3389/fonc.2014.00096
Li, P., & Zhang, L. (2022). Casein kinase 1 in the regulation of cell physiology and brain function. Frontiers in Molecular Neuroscience, 15, 1015360.
https://doi.org/10.3389/fnmol.2022.1015360
Knippschild, U., Wolff, S., Giamas, G., Brockschmidt, C., Wittau, M., Würl, P., & Eismann, T. (2005). The role of the casein kinase 1 (CK1) family in different signaling pathways linked to cancer development. Onkologie, 28(10), 508–514.
https://doi.org/10.1159/000088675
Walton, K. M., Fisher, K., Rubitski, D., Marconi, M., Meng, Q. J., Sládek, M., … & Turek, F. W. (2009). Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. Journal of Pharmacology and Experimental Therapeutics, 330(2), 430–439.