Brexpiprazole and Pimavanserin for Agitation and Psychosis in Alzheimer's Disease
Abstract
Brexpiprazole is a medication used in the treatment of various mental health conditions. It belongs to the class of drugs known as atypical antipsychotics. Brexpiprazole works by affecting the activity of certain neurotransmitters in the brain, including dopamine and serotonin. This medication is primarily prescribed for conditions such as schizophrenia and major depressive disorder. It helps to alleviate symptoms such as hallucinations, delusions, mood disturbances, and cognitive impairments. Brexpiprazole is often used as an adjunctive therapy alongside other treatments or as a standalone option. Studies have shown that Brexpiprazole can help restore the balance of neurotransmitters in the brain, leading to improved symptoms and overall mental well-being.
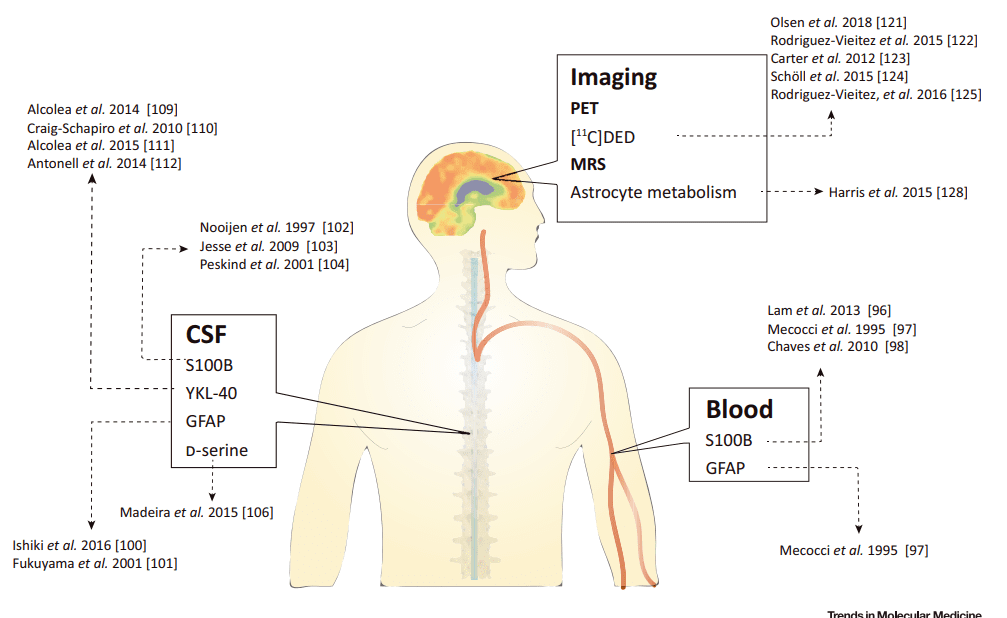
Fig 1. Fluid and Imaging Astroglial Biomarkers in Alzheimer’s Disease [1].
Background on agitation and psychosis in Alzheimer’s disease
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by cognitive decline and memory impairment. However, in addition to these hallmark symptoms, many individuals with Alzheimer’s also experience behavioral and psychological symptoms, including agitation and psychosis.
Agitation refers to a range of behaviors such as restlessness, pacing, verbal or physical aggression, and resistance to care. Psychosis, on the other hand, involves the presence of delusions (firmly held false beliefs) and hallucinations (perceiving things that are not there). These symptoms can significantly impact the quality of life for both individuals with Alzheimer’s and their caregivers.
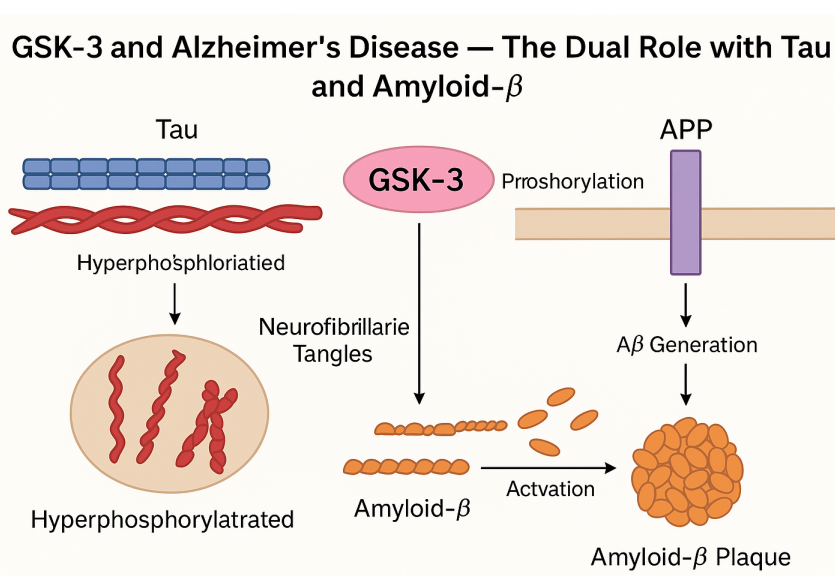
The exact causes of agitation and psychosis in Alzheimer’s disease are not fully understood. However, they are believed to result from a combination of factors, including the progression of the disease, changes in brain chemistry, and the impact of other medical conditions or medications. Additionally, disruptions in neurotransmitter systems, particularly dopamine and serotonin, have been implicated in the development of these symptoms.
Managing agitation and psychosis in Alzheimer’s disease is crucial for the well-being and safety of the affected individuals. Non-pharmacological interventions, such as environmental modifications, behavioral strategies, and caregiver support, are generally recommended as first-line approaches. However, in some cases, pharmacological interventions may be necessary.
The development of new antipsychotic drugs, such as brexpiprazole and pimavanserin, has provided potential treatment options for agitation and psychosis in Alzheimer’s disease. These medications target specific neurotransmitter systems and offer different efficacy and safety profiles compared to older antipsychotics. Understanding the background and context of these symptoms is essential for the appropriate selection and utilization of medications in the management of agitation and psychosis in Alzheimer’s disease.
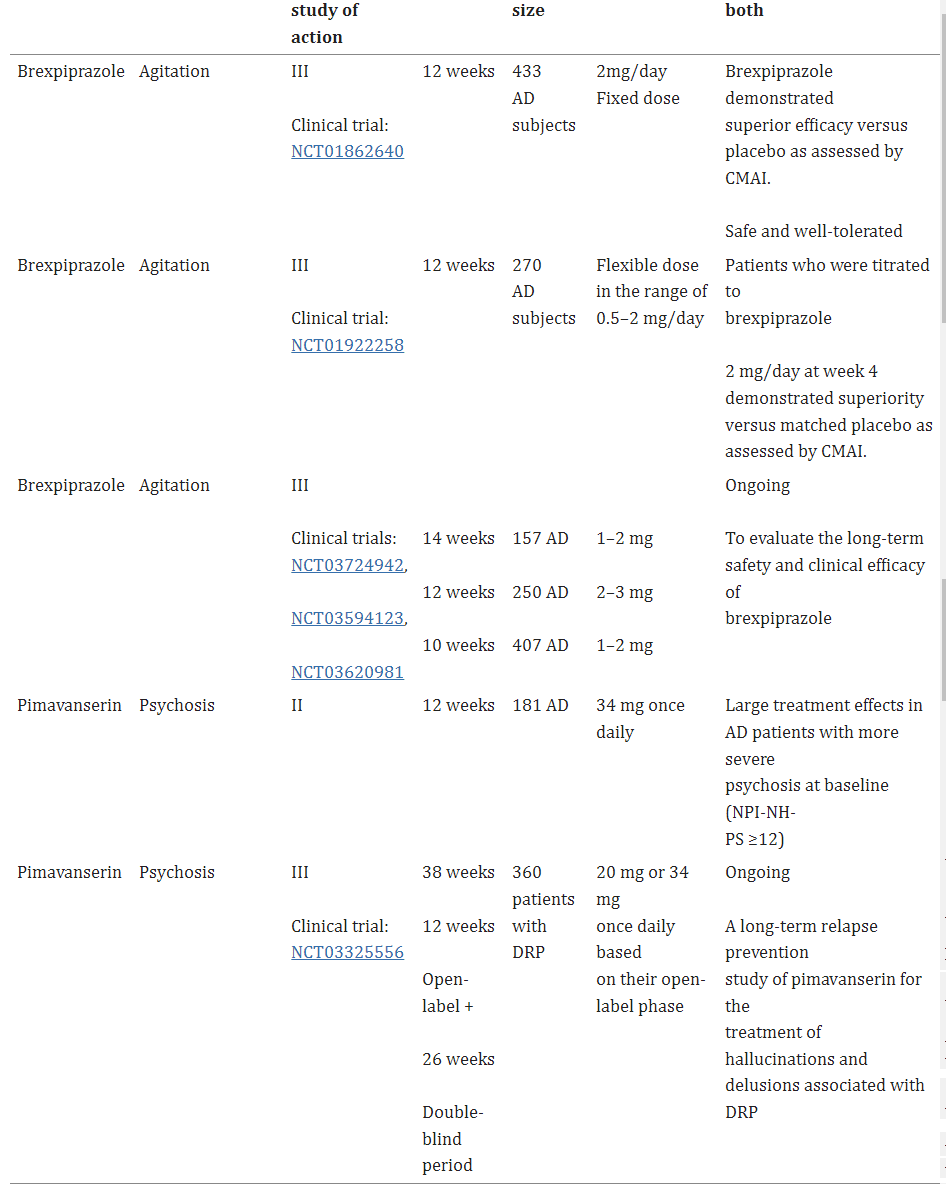
Table 1. Current status of clinical development of brexpiprazole and pimavanserin for the treatment of agitation and psychosis in Alzheimer’s disease [2].
Overview of Brexpiprazole
Description and Mechanism of Action:
Brexpiprazole is a medication with a unique pharmacological profile that contributes to its therapeutic effects. It is classified as an atypical antipsychotic and belongs to the chemical class of benzisoxazole derivatives. The chemical structure of brexpiprazole consists of a dibenzodiazepine core with various substituents.
Brexpiprazole exerts its pharmacological effects through its unique mechanism of action. As a serotonin-dopamine activity modulator (SDAM), it acts as a partial agonist at dopamine D2 and serotonin 5-HT1A receptors and as an antagonist at serotonin 5-HT2A and noradrenaline α1B/α2C receptors. By modulating these neurotransmitter systems, brexpiprazole helps restore the balance of dopamine and serotonin in the brain. The partial agonist activity at D2 receptors contributes to its antipsychotic effects, while the 5-HT1A agonism is believed to enhance its antidepressant properties. These interactions with multiple receptor subtypes make brexpiprazole a unique and versatile medication in the treatment of various mental health conditions.
Pharmacokinetics and Dosage Recommendations:
Brexpiprazole demonstrates favorable pharmacokinetic properties. Following oral administration, it is rapidly absorbed, reaching peak plasma concentrations within a few hours. It has a high bioavailability and is extensively metabolized in the liver, mainly through cytochrome P450 3A4 enzymes. The elimination half-life of brexpiprazole is approximately 91 hours, contributing to its long duration of action.
Dosing recommendations for brexpiprazole vary depending on the condition being treated. In schizophrenia, the recommended dose range typically falls between 1 mg to 4 mg per day. However, the initial dose and subsequent adjustments should be determined by a healthcare professional based on individual patient factors. Dose adjustments may be necessary for patients with hepatic impairment or those taking medications that interact with brexpiprazole’s metabolism.
Efficacy Studies in Alzheimer’s-Related Agitation and Psychosis:
Brexpiprazole has also been studied for its effectiveness in managing agitation and psychosis in Alzheimer’s disease. Clinical trials have demonstrated its efficacy in reducing agitation symptoms and improving overall behavioral outcomes in individuals with Alzheimer’s-related agitation. Additionally, brexpiprazole has shown promising results in reducing psychotic symptoms, such as hallucinations and delusions, in individuals with Alzheimer’s-related psychosis. These studies highlight the potential of brexpiprazole as a treatment option for these challenging symptoms associated with Alzheimer’s disease.
Overall, brexpiprazole’s unique pharmacological profile, favorable pharmacokinetics, and demonstrated efficacy in managing agitation and psychosis in Alzheimer’s disease make it a valuable option in the field of mental health therapeutics.
Overview of Pimavanserin
Pimavanserin is a medication that has gained attention for its specific indication in the treatment of Parkinson’s disease psychosis and Alzheimer’s-related psychosis. It is classified as a selective serotonin inverse agonist and antagonist (SSIA), and its unique pharmacological profile sets it apart from traditional antipsychotic medications.
Description and Mechanism of Action:
Pimavanserin acts primarily as an antagonist at serotonin 5-HT2A receptors, specifically targeting the overactive serotonergic signaling implicated in psychosis. By blocking these receptors, pimavanserin helps to reduce hallucinations and delusions without significantly affecting dopamine receptors, which is important in Parkinson’s disease where maintaining dopaminergic function is crucial.
Pharmacokinetics and Dosage Recommendations:
Pimavanserin is orally administered and exhibits good oral bioavailability. After ingestion, it undergoes metabolism primarily by cytochrome P450 3A4 enzymes. The elimination half-life of pimavanserin is approximately 57 hours. Dosage recommendations for pimavanserin in the treatment of Parkinson’s disease psychosis typically start at 34 mg taken once daily with or without food.
Efficacy Studies in Alzheimer’s-Related Psychosis:
Clinical studies have demonstrated the efficacy of pimavanserin in the treatment of psychosis associated with Alzheimer’s disease. In a pivotal Phase III trial, pimavanserin showed significant improvement in reducing psychotic symptoms compared to placebo, without worsening motor function in patients with Alzheimer’s-related psychosis. Additional studies have further supported these findings, indicating its potential as an effective and well-tolerated treatment option.
Pimavanserin’s unique mechanism of action, primarily targeting 5-HT2A receptors, sets it apart from other antipsychotic medications. Its specificity offers the advantage of reducing the risk of exacerbating motor symptoms in Parkinson’s disease patients. The efficacy studies in Alzheimer’s-related psychosis have provided evidence for its effectiveness and safety, highlighting its potential as a valuable treatment option for patients with these challenging symptoms.
Comparative Analysis of Brexpiprazole and Pimavanserin
Brexpiprazole and pimavanserin are two medications used in the treatment of psychiatric conditions, although they have different primary indications. Despite their distinct therapeutic focuses, there are some similarities and differences between these medications.
Both brexpiprazole and pimavanserin act on the serotonin system, targeting specific receptor subtypes. Brexpiprazole acts as a partial agonist at dopamine D2 and serotonin 5-HT1A receptors, while pimavanserin functions as an antagonist at serotonin 5-HT2A receptors. These interactions aim to restore the balance of neurotransmitters involved in mental health disorders.
In terms of differences, brexpiprazole has a broader range of indications, including schizophrenia and agitation in Alzheimer’s disease, while pimavanserin is specifically approved for Parkinson’s disease psychosis. Efficacy studies have shown that brexpiprazole can effectively reduce symptoms of psychosis and agitation, whereas pimavanserin demonstrates efficacy in treating hallucinations and delusions in Parkinson’s disease psychosis.
Safety and tolerability profiles differ between the two medications. Brexpiprazole is associated with a broader range of potential side effects, including metabolic changes and movement disorders, while pimavanserin is generally well-tolerated with a more favorable side effect profile.
Conclusion
The available evidence supports the efficacy and safety of both brexpiprazole and pimavanserin in their respective therapeutic areas. Clinical studies have demonstrated the effectiveness of brexpiprazole in schizophrenia, agitation in Alzheimer’s disease, and other psychiatric conditions. Pimavanserin has shown promising results in treating Parkinson’s disease psychosis and Alzheimer’s-related psychosis. The evidence suggests that these medications can provide valuable treatment options for patients with specific psychiatric symptoms. On May 11, 2023, the FDA approved a new indication application for brexpiprazole for the treatment of dementia-associated agitation (AAD) in Alzheimer’s disease. This is the first Alzheimer’s agitation drug approved by the FDA.
Rererence
- Carter, S. F., Herholz, K., Rosa-Neto, P., Pellerin, L., Nordberg, A., & Zimmer, E. R. (2019). Astrocyte biomarkers in Alzheimer’s disease. Trends in molecular medicine, 25(2), 77-95.
- Caraci, F., Santagati, M., Caruso, G., Cannavò, D., Leggio, G. M., Salomone, S., & Drago, F. (2020). New antipsychotic drugs for the treatment of agitation and psychosis in Alzheimer’s disease: focus on brexpiprazole and pimavanserin. F1000Research, 9.
- Markovic, M., Gallipani, A., Patel, K. H., & Maroney, M. (2017). Brexpiprazole: a new treatment option for schizophrenia and major depressive disorder. Annals of Pharmacotherapy, 51(4), 315-322.
- Grossberg, G. T., Kohegyi, E., Mergel, V., Josiassen, M. K., Meulien, D., Hobart, M., … & Cummings, J. L. (2020). Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer’s dementia: two 12-week, randomized, double-blind, placebo-controlled trials. The American Journal of Geriatric Psychiatry, 28(4), 383-400.
- Corponi, F., Fabbri, C., Bitter, I., Montgomery, S., Vieta, E., Kasper, S., … & Serretti, A. (2019). Novel antipsychotics specificity profile: A clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. European Neuropsychopharmacology, 29(9), 971-985.
- Frampton, J. E. (2019). Brexpiprazole: a review in schizophrenia. Drugs, 79, 189-200.