Beyond Hypertension: The Expanding Therapeutic Role of Angiotensin Receptor Blockers
Abstract
Angiotensin receptor blockers (ARBs) have long been established as effective treatments for hypertension through selective blockade of the angiotensin II type 1 (AT1) receptor. However, emerging research has revealed their broader therapeutic potential across cardiovascular, neurological, metabolic, and inflammatory diseases. By modulating local renin–angiotensin system activity and shifting signaling toward protective AT2 receptor pathways, ARBs exert anti-inflammatory, anti-fibrotic, and neuroprotective effects. This article explores their evolving role, from cardiovascular protection to precision medicine applications, highlighting opportunities for drug repurposing in multi-system health management.
ARBs Step Out of the Hypertension Box
For decades, angiotensin receptor blockers (ARBs) have been a mainstay treatment for hypertension, acting by selectively blocking the angiotensin II type 1 (AT1) receptor. This mechanism prevents vasoconstriction, reduces aldosterone secretion, and lowers blood pressure—making ARBs a trusted first-line option for cardiovascular care. However, in recent years, the scope of ARB therapy has expanded far beyond its original purpose, revealing a promising role in disease modulation across multiple organ systems.
At the core of this expansion lies the understanding that the renin–angiotensin system (RAS) is not limited to systemic blood pressure regulation. Local tissue RAS networks operate in the heart, brain, kidneys, pancreas, and immune system, influencing processes like inflammation, fibrosis, oxidative stress, and cellular survival. By blocking AT1 receptors, ARBs not only reduce hemodynamic stress but also shift angiotensin II signaling toward the angiotensin II type 2 (AT2) receptor pathway, which is associated with vasodilation, anti-inflammatory, anti-fibrotic, and neuroprotective effects (Saavedra, 2021).
This shift in therapeutic perspective has opened the door to exploring ARBs in conditions such as heart failure, atrial fibrillation, chronic kidney disease, metabolic syndrome, and even neurodegenerative disorders like Alzheimer’s and Parkinson’s disease. The broad spectrum of effects is partly due to ARBs’ ability to modulate oxidative stress pathways, suppress pro-inflammatory cytokines, and enhance endothelial function (Benigni et al., 2010).
Drug repurposing—the application of existing medications for new therapeutic purposes—has emerged as a cost-effective strategy for accelerating medical innovation. ARBs are prime candidates for repurposing due to their excellent safety profile, long-term tolerability, and well-understood pharmacology. Furthermore, their oral bioavailability and once-daily dosing make them practical for chronic use in diverse patient populations.
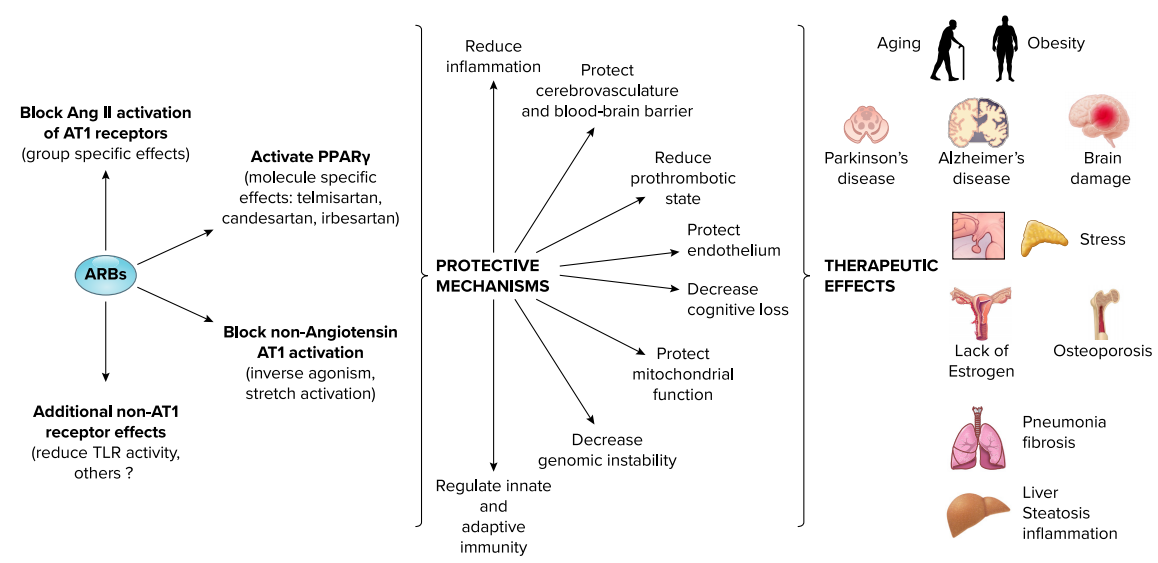
FIGURE 1. Mechanisms of action, principal protective effects, and influence of angiotensin receptor blocker (ARB) treatment beyond cardiovascular, renal, and metabolic disorders.
As emerging clinical and preclinical data accumulate, the narrative around ARBs is evolving from “blood pressure drugs” to multi-target pharmacological agents. This broader view encourages both clinicians and researchers to rethink how these well-known medications might contribute to preventive medicine, multi-morbidity management, and personalized therapy in the years ahead.
ARBs in Cardiovascular Protection and Beyond
While angiotensin receptor blockers (ARBs) were initially developed for hypertension management, accumulating evidence has demonstrated their broad cardiovascular protective effects that extend well beyond blood pressure control. By antagonizing angiotensin II type 1 (AT1) receptors, ARBs not only reduce vasoconstriction and fluid retention but also modulate vascular inflammation, oxidative stress, and structural remodeling—processes that are central to many cardiovascular pathologies (Burnier & Brunner, 2000).
Endothelial Function and Vascular Health
One of the pivotal benefits of ARBs is their ability to restore endothelial function, which is often impaired in cardiovascular disease. Endothelial dysfunction promotes atherosclerosis and thrombosis, but ARBs enhance nitric oxide bioavailability and decrease reactive oxygen species production. This leads to improved vascular tone, reduced platelet aggregation, and greater vascular resilience (Dharmashankar & Widlansky, 2010).
Heart Failure and Post-MI Protection
In heart failure and post-myocardial infarction patients, ARBs reduce afterload, prevent maladaptive left ventricular remodeling, and improve cardiac output. Large clinical trials, such as VALIANT, have shown that ARBs like valsartan can be as effective as ACE inhibitors in improving survival and reducing hospitalizations when ACE inhibitors are not tolerated (Pfeffer et al., 2003).
Atrial Fibrillation and Anti-arrhythmic Potential
Emerging data suggest that ARBs may lower the incidence of atrial fibrillation (AF), particularly in hypertensive and heart failure patients. This effect is linked to their ability to reduce atrial fibrosis and inflammation, which are key contributors to AF development (Healey et al., 2005). ARBs’ anti-arrhythmic potential could make them an important adjunct in rhythm control strategies.
Anti-inflammatory and Anti-thrombotic Roles
Beyond hemodynamic effects, ARBs reduce the expression of pro-inflammatory cytokines and adhesion molecules, thereby mitigating vascular inflammation. Their antithrombotic properties, possibly related to endothelial protection and reduced platelet activation, contribute to their overall cardioprotective profile.
In summary, ARBs act on multiple fronts—improving vascular health, reducing pathological remodeling, and preventing arrhythmias—making them valuable agents in the comprehensive management of cardiovascular disease.
Neuroprotective Potential — ARBs in Brain Health
The brain possesses its own local renin–angiotensin system (RAS), which plays a crucial role in regulating cerebral blood flow, neuronal survival, and neuroinflammation. While angiotensin II acting on AT1 receptors can promote oxidative stress, neuroinflammation, and neuronal apoptosis, blocking these receptors with angiotensin receptor blockers (ARBs) can shift signaling toward AT2 receptor pathways, known for neuroprotective and vasodilatory effects (Saavedra, 2012).
Reducing Neuroinflammation
Neuroinflammation is a hallmark of many neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). By inhibiting AT1 receptors, ARBs suppress pro-inflammatory cytokines such as TNF-α and IL-6, reduce activation of microglia, and lower oxidative stress markers in brain tissue. This anti-inflammatory effect may slow neurodegenerative progression and protect neuronal networks (Villapol & Saavedra, 2015).
Cognitive Protection and Dementia Risk Reduction
Several observational and interventional studies suggest that ARB use is associated with slower cognitive decline and a reduced risk of dementia in hypertensive patients. Mechanistically, ARBs improve endothelial function in cerebral vessels, enhancing blood flow and oxygen delivery to brain tissue. Some ARBs, such as telmisartan, also cross the blood–brain barrier, allowing direct neuronal protection and modulation of signaling pathways involved in memory and learning (Sink et al., 2009).
Stroke Recovery and Neurovascular Health
In acute and post-stroke settings, ARBs may offer benefits beyond blood pressure control. They help reduce cerebral edema, protect the blood–brain barrier, and promote angiogenesis in peri-infarct regions, improving functional recovery. Animal studies have shown that ARBs can limit neuronal death in ischemic models through anti-apoptotic signaling.
Potential in Psychiatric Disorders
Emerging research links RAS hyperactivation to mood and anxiety disorders. ARBs’ anti-inflammatory and neurovascular benefits may extend to psychiatric health, with preliminary evidence suggesting improvements in stress-related neuropathology.
In summary, the neuroprotective role of ARBs stems from their ability to counteract harmful AT1-mediated processes and promote protective AT2 signaling. This positions ARBs as potential therapeutic tools not only for cardiovascular and metabolic health but also for brain aging and neurodegenerative disease prevention.
ARBs in Metabolic and Inflammatory Disorders
Although angiotensin receptor blockers (ARBs) are best known for their cardiovascular benefits, their influence extends into the realms of metabolic health and systemic inflammation. This is largely because the renin–angiotensin system (RAS) is active in tissues beyond the vascular system, including the pancreas, adipose tissue, and immune cells. Overactivation of the RAS in these tissues contributes to insulin resistance, chronic inflammation, and fibrosis—key mechanisms underlying many chronic diseases.
Improving Insulin Sensitivity
In metabolic syndrome and type 2 diabetes, elevated angiotensin II signaling through the AT1 receptor promotes oxidative stress, impairs insulin signaling, and reduces glucose uptake in skeletal muscle. ARBs counteract these effects by blocking AT1 receptors, thereby improving insulin sensitivity and glucose metabolism. Some ARBs, such as telmisartan, have partial peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist activity, which further enhances their insulin-sensitizing properties.
Protecting Against Diabetic Complications
In diabetic nephropathy—a major microvascular complication—ARBs lower intraglomerular pressure, reduce proteinuria, and inhibit fibrosis in kidney tissue. This renoprotective effect goes beyond blood pressure control and is linked to suppression of inflammatory mediators and fibrotic pathways. As a result, ARBs are often recommended as first-line agents for patients with diabetes and kidney involvement.
Anti-inflammatory Properties
ARBs modulate immune responses by reducing the production of pro-inflammatory cytokines (e.g., TNF-α, IL-6) and adhesion molecules that drive chronic inflammation. This has led to research into their potential role in autoimmune conditions such as rheumatoid arthritis and multiple sclerosis. By decreasing inflammatory cell infiltration into tissues, ARBs may help limit disease progression in these contexts.
Emerging Clinical Implications
Beyond traditional indications, ARBs have been investigated for their potential in non-alcoholic fatty liver disease (NAFLD), where they may reduce hepatic inflammation and fibrosis. Preclinical studies have also explored their utility in chronic obstructive pulmonary disease (COPD), where inflammation-driven tissue remodeling plays a central role. While more human data are needed, these findings support the concept of ARBs as multi-system modulators.
In summary, the anti-inflammatory, anti-fibrotic, and metabolic effects of ARBs position them as promising agents for managing complex, multi-organ disorders where chronic inflammation and metabolic dysfunction intersect.
Future Directions — Repurposing ARBs for Precision Medicine
As research into the renin–angiotensin system (RAS) expands, angiotensin receptor blockers (ARBs) are increasingly recognized as multi-system modulators with the potential to be repurposed well beyond traditional cardiovascular care. The next wave of innovation lies in leveraging their molecular versatility, favorable safety profile, and multi-target mechanisms for precision medicine.
Multi-Disease Applications
ARBs’ anti-inflammatory, anti-fibrotic, and endothelial-protective actions open the door to their use in diverse conditions. Ongoing studies are exploring ARBs in neurodegenerative diseases such as Alzheimer’s and Parkinson’s, chronic kidney disease, metabolic syndrome, and autoimmune disorders. Their ability to modulate local RAS activity in various organs positions them as candidates for multi-disease prevention and management strategies.
Role in Emerging Infectious Disease
During the COVID-19 pandemic, interest in ARBs surged due to their interaction with ACE2 regulation—a receptor also exploited by SARS-CoV-2. Although the clinical data remain mixed, preclinical evidence suggests ARBs could mitigate virus-induced inflammation and organ injury without worsening viral entry. This area continues to attract research, particularly for post-viral inflammatory syndromes.
Precision and Pharmacogenomics
One of the most promising frontiers is the use of genomic profiling to predict individual responses to ARB therapy. Variants in RAS-related genes may influence drug efficacy, side-effect profiles, and cross-disease benefits. Tailoring ARB choice and dosage based on genetic markers could optimize therapeutic outcomes, reduce adverse effects, and enable their use in niche populations.
Combination Therapies
Another avenue is combining ARBs with other targeted agents to enhance efficacy. For example, pairing ARBs with anti-inflammatory biologics or metabolic modulators could yield synergistic benefits in complex diseases. In oncology, ARBs are being tested alongside chemotherapy and immunotherapy to improve drug delivery and tumor microenvironment modulation.
The Road Ahead
For ARBs to achieve their full potential in precision medicine, more randomized controlled trials are needed in non-hypertensive indications. These should explore long-term safety, dose optimization, and patient stratification strategies. With their well-documented safety record and expanding mechanistic understanding, ARBs stand poised to become central players in the shift toward personalized, multi-target pharmacology.
References
Benigni, A., Cassis, P., & Remuzzi, G. (2010). Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Molecular Medicine, 2(7), 247–257.
https://doi.org/10.1002/emmm.201000080
Kobori, H., Nangaku, M., Navar, L. G., & Nishiyama, A. (2007). The intrarenal renin–angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacological Reviews, 59(3), 251–287.
https://doi.org/10.1124/pr.59.3.3
Saavedra, J. M. (2021). Angiotensin receptor blockers are not just for hypertension anymore. International Journal of Molecular Sciences, 22(12), 6478.
https://doi.org/10.3390/ijms22126478
Sharma, A. M., & Janke, J. (2014). Renin–angiotensin system inhibitors and prevention of type 2 diabetes mellitus: A meta-analysis. Diabetologia, 57(12), 2550–2559.
https://doi.org/10.1007/s00125-014-3394-5
Burnier, M., & Brunner, H. R. (2000). Angiotensin II receptor antagonists. The Lancet, 355(9204), 637–645.
https://doi.org/10.1016/S0140-6736(99)10365-1
Dharmashankar, K., & Widlansky, M. E. (2010). Vascular endothelial function and hypertension: Insights and directions. Current Hypertension Reports, 12(6), 448–455.
https://doi.org/10.1007/s11906-010-0150-2
Pfeffer, M. A., McMurray, J. J., Velazquez, E. J., Rouleau, J. L., Køber, L., Maggioni, A. P., … & Solomon, S. D. (2003). Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. New England Journal of Medicine, 349(20), 1893–1906.
https://doi.org/10.1056/NEJMoa032292
Healey, J. S., Baranchuk, A., Crystal, E., Morillo, C. A., Garfinkle, M., Yusuf, S., & Connolly, S. J. (2005). Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: A meta-analysis. Journal of the American College of Cardiology, 45(11), 1832–1839.
https://doi.org/10.1016/j.jacc.2004.11.070
Saavedra, J. M. (2012). Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clinical Science, 123(10), 567–590.
https://doi.org/10.1042/CS20120078
Sink, K. M., Leng, X., Williamson, J., Kritchevsky, S. B., Yaffe, K., Kuller, L., … & Lopez, O. L. (2009). Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension. Archives of Internal Medicine, 169(13), 1195–1202.
https://doi.org/10.1001/archinternmed.2009.175
Villapol, S., & Saavedra, J. M. (2015). Neuroprotective effects of angiotensin receptor blockers. American Journal of Hypertension, 28(3), 289–299.
https://doi.org/10.1093/ajh/hpu197
Kehoe, P. G., Wong, S., Al Mulhim, N., Palmer, L. E., & Miners, J. S. (2016). Angiotensins in Alzheimer’s disease — friend or foe? Trends in Neurosciences, 39(10), 694–706.
https://doi.org/10.1016/j.tins.2016.08.001
alra, S., Kalra, B., & Agrawal, N. (2011). Combination therapy in hypertension: An update on angiotensin receptor blockers and hydrochlorothiazide. Vascular Health and Risk Management, 7, 565–573.
https://doi.org/10.2147/VHRM.S20697
Ferrario, C. M., Jessup, J., & Chappell, M. C. (2005). Blockade of angiotensin II type 1 receptors ameliorates angiotensin-(1-12)-mediated hypertension and end-organ damage. Hypertension, 45(5), 975–981.