AZD0022 Explained: How AstraZeneca’s New Drug Could Transform Cancer Treatment
Abstract
AZD0022 is an investigational small-molecule inhibitor developed by AstraZeneca that targets the KRAS G12D mutation—an oncogenic driver found in pancreatic, colorectal, and non-small cell lung cancers. As a first-in-class inhibitor, AZD0022 addresses a significant unmet need in precision oncology by offering a mutation-specific approach to a historically undruggable target. Early clinical trial data suggest promising safety and efficacy, particularly when combined with MEK inhibitors or immunotherapy agents. This blog explores the scientific rationale behind AZD0022, its mechanism of action, current clinical development, and its potential to reshape treatment strategies for KRAS-driven cancers. With ongoing research and a growing focus on tumor-agnostic therapies, AZD0022 may represent a significant breakthrough in the treatment of mutation-specific cancers.
Introduction
In the evolving landscape of targeted oncology, AZD0022 has emerged as one of the most anticipated drug candidates in 2025. Developed by AstraZeneca, AZD0022 is a next-generation small molecule inhibitor designed to target the KRAS G12D mutation, a prevalent oncogenic driver found in some of the deadliest cancers—including pancreatic, colorectal, and non-small cell lung cancer (NSCLC).
While past efforts to drug KRAS proteins have been notoriously challenging due to their smooth molecular surfaces and lack of deep binding pockets, recent breakthroughs—especially with KRAS G12C inhibitors—have reinvigorated interest in this field. However, G12D, which is even more common in certain tumors, has remained largely elusive. AZD0022 represents a significant scientific and clinical milestone as it directly targets this specific mutation, potentially offering new hope for patients with limited treatment options.
Early-stage preclinical trials have demonstrated promising tumor suppression activity and manageable toxicity profiles. The drug is currently under investigation in a Phase I clinical trial that assesses its safety and therapeutic potential both as a monotherapy and in combination with other agents such as MEK inhibitors.
As presentations at AACR 2025 and reports from Drug Hunter have highlighted, AZD0022 is not just another molecule in development—it’s the vanguard of a new class of KRAS G12D-specific therapies. Its emergence underscores AstraZeneca’s deepening focus on precision oncology and reinforces the broader industry shift toward mutation-specific cancer treatments.
What is KRAS G12D and Why Does it Matter?
The KRAS gene, a member of the RAS family, encodes a GTPase that plays a crucial role in regulating cell proliferation, differentiation, and survival. Mutations in this gene are among the most common genetic alterations observed in cancer. One particular variant, KRAS G12D, results from a glycine-to-aspartic acid substitution at codon 12, leading to constitutive activation of the KRAS protein and persistent downstream signaling through pathways such as MAPK/ERK and PI3K/AKT.
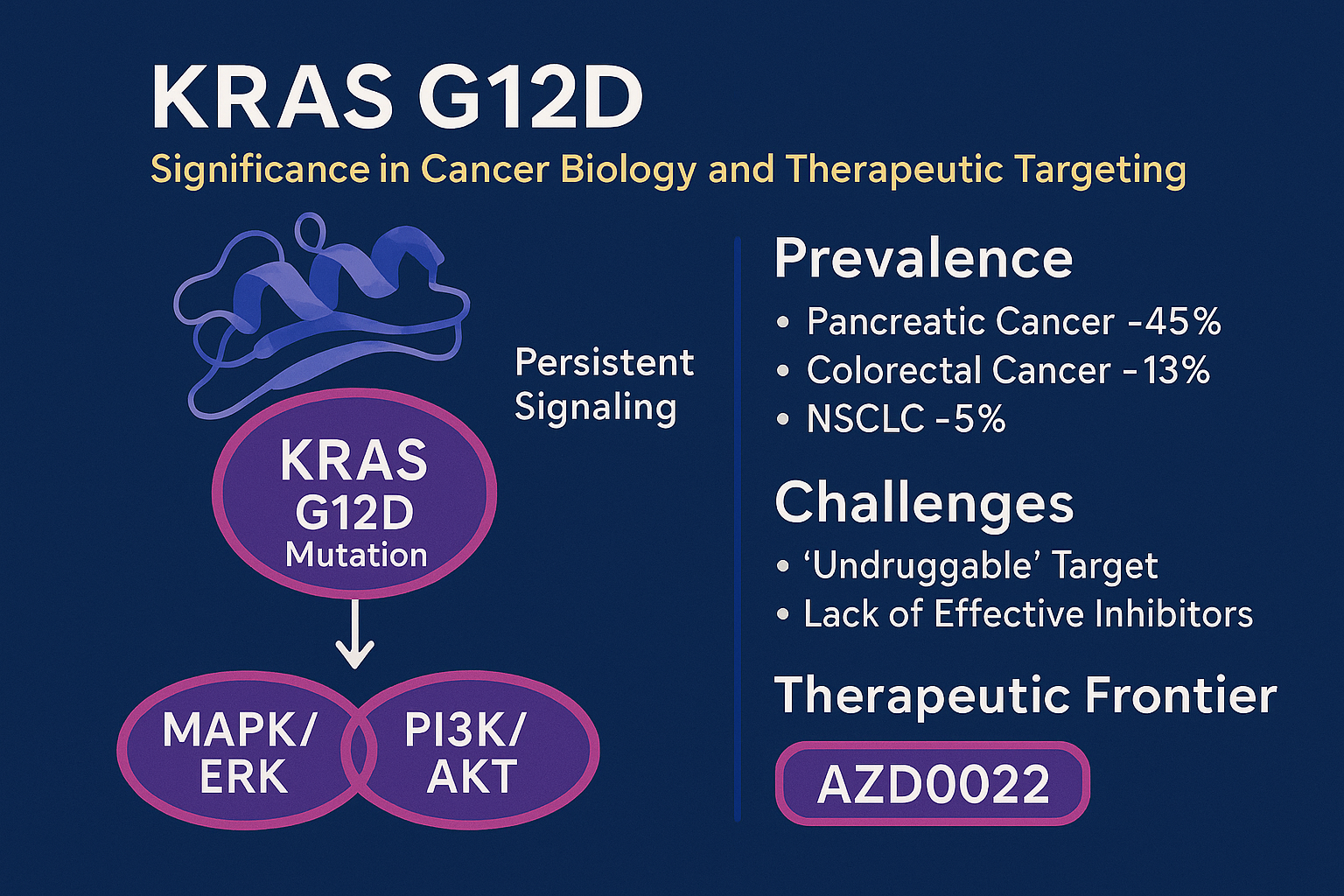
KRAS G12D mutations are particularly prevalent in pancreatic ductal adenocarcinoma (up to 45%), colorectal cancer (around 13%), and non-small cell lung cancer (NSCLC, approximately 5%). These cancers are often aggressive, difficult to treat, and exhibit poor survival outcomes, in part due to the lack of effective targeted therapies for KRAS G12D.
Historically, KRAS mutations have been labeled as “undruggable” due to the protein’s smooth surface and high affinity for GTP/GDP, which complicates small molecule binding. Although some therapeutic progress has been made with KRAS G12C inhibitors like sotorasib, these are ineffective against G12D mutations due to structural differences.
This unmet clinical need has driven a wave of interest in developing KRAS G12D-selective inhibitors, and AZD0022 is at the forefront of this effort. The importance of targeting KRAS G12D lies not only in its high prevalence in certain tumors but also in its role in mediating resistance to existing therapies.
AZD0022 – Mechanism of Action
AZD0022 is a covalent, small-molecule inhibitor engineered by AstraZeneca to selectively target the KRAS G12D mutant protein, which drives oncogenesis through aberrant signal transduction. Unlike earlier approaches that attempted to inhibit upstream or downstream effectors of the KRAS pathway, AZD0022 binds directly to the KRAS G12D protein, interfering with its ability to cycle between active (GTP-bound) and inactive (GDP-bound) states.
The specificity of AZD0022 stems from its ability to recognize the unique structural features of the G12D mutation. This mutation introduces a negatively charged aspartate residue that alters the protein’s conformation, which AZD0022 exploits for selective binding. The compound forms an irreversible covalent bond within the switch II pocket of the mutant KRAS protein, effectively locking it in an inactive conformation and preventing activation of downstream pathways like RAF-MEK-ERK.
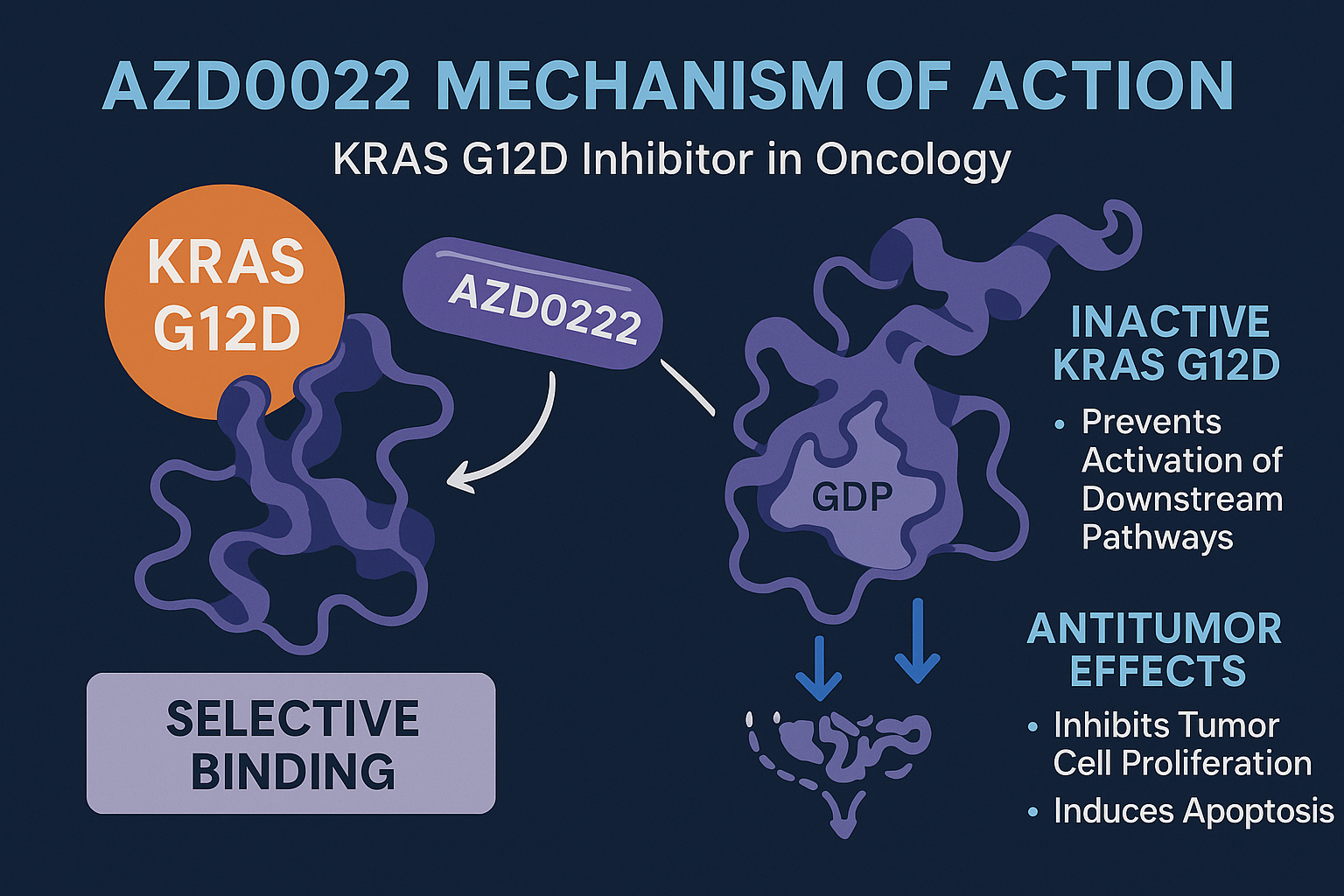
By directly suppressing KRAS G12D signaling, AZD0022 can inhibit tumor cell proliferation and induce apoptosis in cancer cells dependent on this mutation. Preclinical studies have shown robust inhibition of KRAS G12D-driven tumor growth in mouse models, with minimal off-target toxicity.
Importantly, AZD0022 is being evaluated not only as a monotherapy but also in combination with other agents such as MEK inhibitors or immune checkpoint inhibitors, aiming to enhance its therapeutic impact and counter potential resistance mechanisms.
This precise, mutation-specific approach aligns with the current trends in precision oncology, offering a highly targeted and potentially less toxic alternative to traditional chemotherapy or broadly acting kinase inhibitors.
Clinical Development of AZD0022
AZD0022 is currently in the early stages of clinical evaluation as a first-in-class, KRAS G12D-specific inhibitor. Its development is being spearheaded by AstraZeneca, a global leader in precision oncology. Following encouraging results in preclinical studies, AZD0022 advanced into Phase I clinical trials in late 2024, focusing on patients with advanced solid tumors that harbor KRAS G12D mutations.
The ongoing Phase I study (NCT06235529) is designed as a dose-escalation and dose-expansion trial, assessing the safety, pharmacokinetics, and preliminary efficacy of AZD0022. The trial includes patients with pancreatic ductal adenocarcinoma, colorectal cancer, and NSCLC, as these malignancies frequently carry KRAS G12D mutations. Importantly, the study also explores combination regimens, evaluating AZD0022 alongside other pathway inhibitors such as MEK inhibitors and immunotherapies.
Data presented at AACR 2025 highlighted that AZD0022 has shown early signs of anti-tumor activity, including partial responses and prolonged stable disease in some patients. The drug exhibited a manageable safety profile, with the most common adverse events being mild to moderate gastrointestinal effects and fatigue. These initial results support further investigation into optimized dosing and therapeutic combinations.
As AZD0022 continues to progress through the clinical pipeline, it has garnered attention from oncologists, investors, and pharmaceutical strategists alike. It represents not only a potential treatment for hard-to-treat tumors but also a validation of mutation-specific drug design targeting KRAS variants previously deemed “undruggable.”
AZD0022 and Target Cancers
AZD0022 is being developed to address a critical need in treating cancers driven by KRAS G12D mutations, which are common across several aggressive tumor types. These include pancreatic ductal adenocarcinoma (PDAC), colorectal cancer (CRC), and non-small cell lung cancer (NSCLC)—each of which presents significant therapeutic challenges and limited survival rates.
In PDAC, KRAS mutations are detected in over 90% of cases, with KRAS G12D comprising nearly 45%. The disease is notorious for its resistance to chemotherapy and its poor prognosis. In colorectal cancer, approximately 13% of cases harbor the G12D variant, contributing to resistance to EGFR inhibitors and leading to aggressive tumor progression. In NSCLC, KRAS G12D occurs in a smaller percentage (~5%) but remains significant due to limited treatment options outside of immunotherapy.
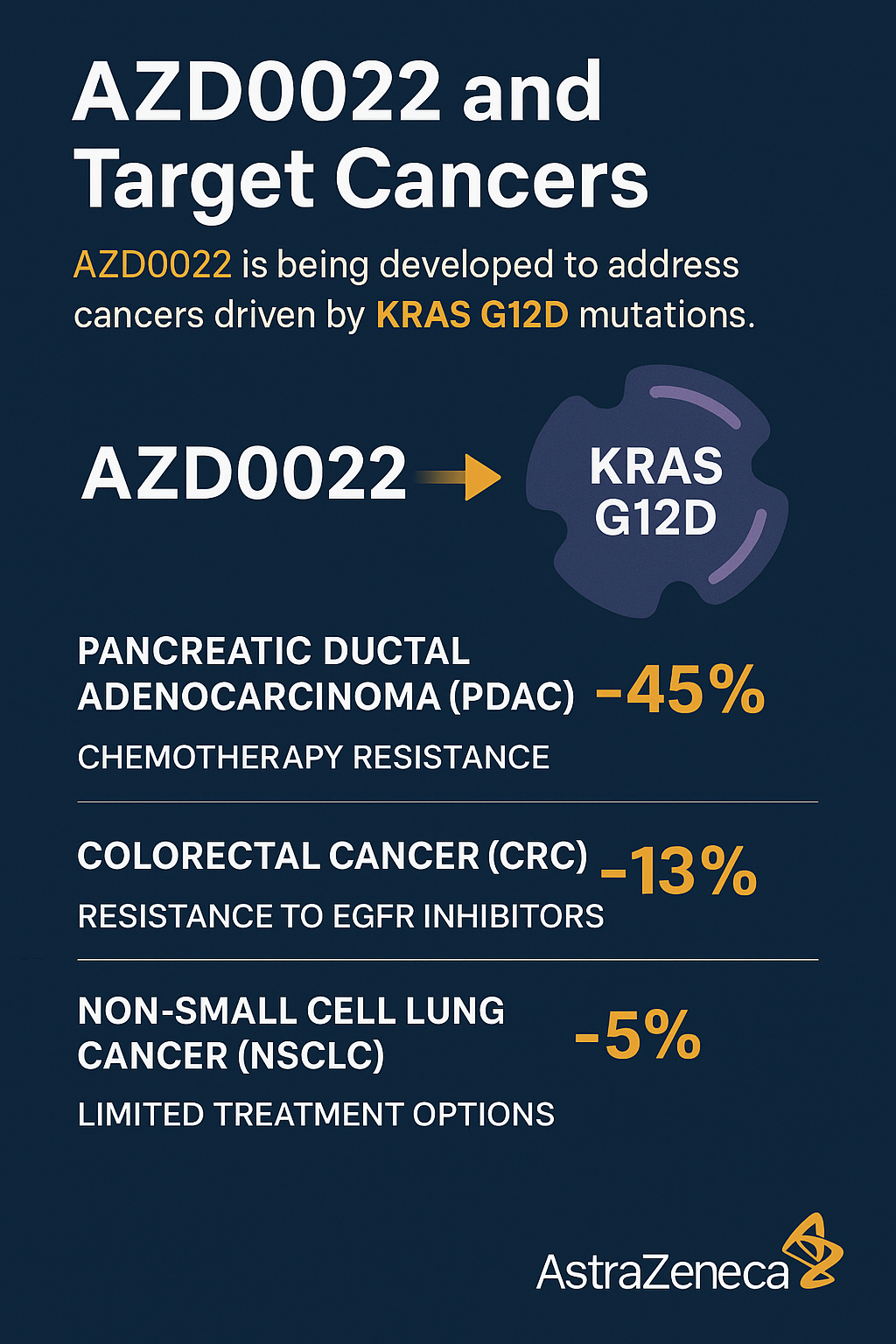
AZD0022 directly targets this mutation, offering a precision-based approach that could overcome resistance to traditional therapies. Its mutation specificity minimizes off-target effects and holds promise for synergistic application with other agents—such as checkpoint inhibitors or downstream MAPK pathway blockers.
Additionally, by focusing on KRAS G12D across multiple tumor types, AZD0022 exemplifies the tumor-agnostic paradigm in oncology drug development, where drugs are approved based on genetic mutations rather than tumor location. This positions AZD0022 as a potential breakthrough across a spectrum of malignancies, mirroring the approval path of other targeted therapies like pembrolizumab and larotrectinib.
The success of AZD0022 could reshape treatment strategies and provide long-awaited hope for patients with these mutation-driven cancers.
AZD0022 in Combination Therapy
While AZD0022 demonstrates promising monotherapy activity in cancers with KRAS G12D mutations, its full therapeutic potential may be unlocked through combination strategies. Like other targeted therapies, single-agent KRAS inhibitors face the challenge of adaptive resistance and tumor heterogeneity. Therefore, combining AZD0022 with other agents is a central theme in AstraZeneca’s development strategy.
Early preclinical studies and clinical trial protocols have already explored AZD0022 in tandem with MEK inhibitors, which act downstream in the RAS-RAF-MEK-ERK pathway. By inhibiting two sequential nodes in the signaling cascade, this dual approach may help overcome compensatory reactivation mechanisms that often limit the durability of single-agent responses.
Additionally, combination with immune checkpoint inhibitors (ICIs) such as anti-PD-1 or anti-PD-L1 antibodies is a focus of investigation. KRAS-mutant tumors often exhibit immunosuppressive microenvironments, and blocking KRAS G12D could enhance tumor immunogenicity, making ICIs more effective. Preclinical mouse models suggest synergistic tumor regression when AZD0022 is paired with immunotherapy, particularly in PDAC and NSCLC models.
AstraZeneca is also considering triplet regimens, including AZD0022 + MEK inhibitor + anti-PD-1 antibody, which aim to modulate both tumor cell signaling and the immune milieu simultaneously.
These combination strategies are being actively evaluated in expansion arms of ongoing Phase I trials, and future clinical trial designs are likely to include adaptive combination frameworks based on biomarker response and resistance profiling.
Combination therapy could ultimately expand the use of AZD0022 beyond monogenic-driven tumors and allow broader patient inclusion while increasing clinical efficacy and long-term disease control.
Conclusion
AZD0022 represents a major advancement in the pursuit of targeted therapies for cancers driven by KRAS G12D mutations, one of the most challenging oncogenic alterations in modern oncology. Developed by AstraZeneca, this mutation-specific inhibitor not only provides a new treatment avenue for aggressive cancers such as pancreatic, colorectal, and non-small cell lung cancers, but also demonstrates the growing sophistication of precision oncology.
Its early success in preclinical models and promising data from ongoing clinical trials suggest that AZD0022 could become a cornerstone therapy, especially when used in combination with MEK inhibitors or immunotherapies. As a first-in-class KRAS G12D inhibitor, it marks a turning point in addressing previously “undruggable” targets, which opens the door for similar innovations targeting other KRAS variants.
Moreover, AZD0022 reflects a broader shift in cancer treatment strategy—from generalized chemotherapies to tumor-agnostic, mutation-guided therapies. If ongoing clinical trials continue to show efficacy and tolerability, AZD0022 may significantly reshape the treatment landscape for mutation-driven cancers and improve outcomes for patients who have long faced limited options.
As research progresses, AZD0022 will be closely watched by clinicians, researchers, and investors alike. It stands not just as a molecule, but as a milestone in targeted cancer therapy innovation.
References
AstraZeneca. (2025). AZD0022 clinical trial overview.
https://clinicaltrials.eu/trial/azd0022
Drug Hunter. (2025). New drugs on the horizon: AACR Chicago 2025 highlights.
https://drughunter.com/articles/aacr-chicago-2025-new-drugs-on-the-horizon
MedChemExpress. (2025). AZD0022: KRAS G12D inhibitor.
https://www.medchemexpress.com/azd0022.html
American Association for Cancer Research (AACR). (2025). Annual Meeting News: New Therapeutics Spotlight.




