Asciminib: A Novel Therapy for Chronic Myeloid Leukemia (CML)
Abstract
Asciminib (also known as ABL001) is a small-molecule tyrosine kinase inhibitor (TKI) that targets the BCR-ABL fusion protein, which is associated with chronic myeloid leukemia (CML). Asciminib works by binding to a specific site on the BCR-ABL protein, known as the myristoyl binding pocket, which is separate from the ATP-binding site targeted by other TKIs such as imatinib. The unique mechanism of action of Asciminib makes it potentially effective against CML cells that have developed resistance to other TKIs, such as imatinib or dasatinib. In clinical trials, Asciminib has demonstrated promising results as a treatment for patients with CML who have failed other therapies, including those with BCR-ABL mutations that confer resistance to other TKIs.
Chronic Myeloid Leukemia (CML)
Chronic myeloid leukemia (CML) is a type of cancer that affects the blood and bone marrow. It is a rare disease that occurs most commonly in adults, but can also occur in children. CML is characterized by the presence of an abnormal chromosome called the Philadelphia chromosome (Ph), which is created when two normal chromosomes in a cell break and swap genetic material. The Ph chromosome contains a fusion gene called BCR-ABL, which produces a protein that is responsible for the uncontrolled growth and division of white blood cells.
Symptoms of CML can vary depending on the stage of the disease, but may include fatigue, weakness, weight loss, fever, and enlarged spleen. CML is typically diagnosed through a combination of blood tests, bone marrow biopsy, and genetic testing.
Current treatment options for CML and the limitations of existing therapies
The current standard of care for chronic myeloid leukemia (CML) involves the use of tyrosine kinase inhibitors (TKIs), such as imatinib, dasatinib, and nilotinib. While these drugs have been successful in inducing remission in many patients with CML, there are several limitations to their use.
One major issue is the development of drug resistance, which can occur through various mechanisms including point mutations in the BCR-ABL kinase domain. In some cases, patients may also experience intolerable side effects from TKIs, such as fatigue, nausea, and muscle cramps. Another limitation of TKIs is their inability to completely eradicate leukemia stem cells, which may be responsible for disease relapse. Furthermore, the high cost of TKIs can pose a significant financial burden for patients and healthcare systems.
Therefore, there is a need for new treatment options for CML, such as Asciminib, an investigational agent that targets BCR-ABL1 via a unique allosteric mechanism. The development of such novel therapies could potentially overcome the limitations of existing treatments and improve the outcomes for patients with CML.
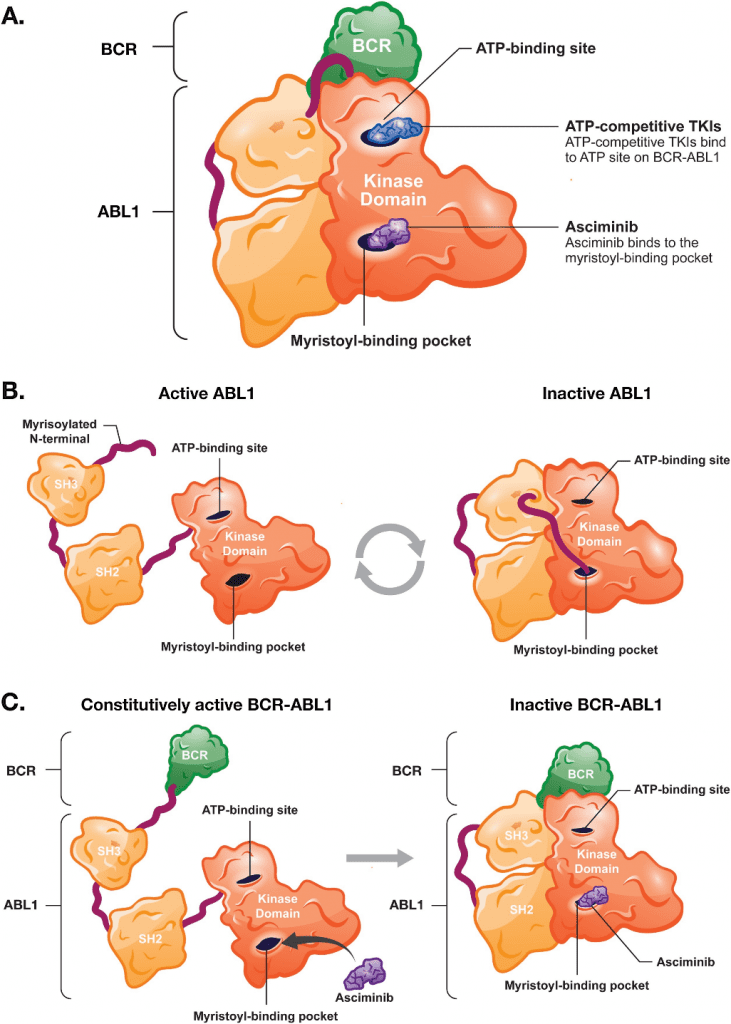
Fig 1. Asciminib’s unique mechanism of action is distinct from ATP-competitive TKIs.[2]
Mechanism of action of Asciminib as an allosteric inhibitor of BCR-ABL1
Asciminib is a novel allosteric inhibitor of BCR-ABL1, a fusion protein that drives the development of chronic myeloid leukemia (CML). The drug works by binding to a site on the BCR-ABL1 kinase domain that is distinct from the ATP-binding site, which is targeted by existing tyrosine kinase inhibitors (TKIs). This unique mechanism of action allows Asciminib to overcome some of the limitations of current therapies, such as drug resistance and intolerance.
When Asciminib binds to the allosteric site on BCR-ABL1, it induces a conformational change in the protein, leading to the inhibition of its kinase activity. This results in the suppression of downstream signaling pathways that promote the uncontrolled growth and division of CML cells. Unlike ATP-competitive TKIs, Asciminib is not affected by mutations in the BCR-ABL1 kinase domain that confer resistance to existing therapies. Additionally, Asciminib has been shown to have a favorable safety profile in clinical trials, with fewer adverse events reported compared to traditional TKIs.
Preclinical studies of Asciminib
Preclinical studies of Asciminib, a novel allosteric inhibitor of BCR-ABL1, have demonstrated its potential as a promising therapy for the treatment of chronic myeloid leukemia (CML). In vitro studies have shown that Asciminib is highly selective for BCR-ABL1 and has potent inhibitory effects on the kinase activity of this fusion protein. In addition, Asciminib has been found to overcome resistance to traditional tyrosine kinase inhibitors (TKIs) in preclinical models of CML.
In vivo studies have also demonstrated the efficacy of Asciminib in reducing tumor growth and inducing remission in mouse models of CML. Furthermore, combination therapy with Asciminib and other TKIs has shown synergistic effects in preclinical studies, suggesting the potential for improved outcomes in patients with CML.
Overall, preclinical studies of Asciminib have provided strong evidence of its potential as a novel therapeutic option for CML. These findings have led to the initiation of clinical trials to evaluate the safety and efficacy of Asciminib in human patients with CML, with promising early results reported. Ongoing research will continue to explore the optimal use of Asciminib in the management.
Table 1. Ongoing clinical trials with Asciminib.[4]
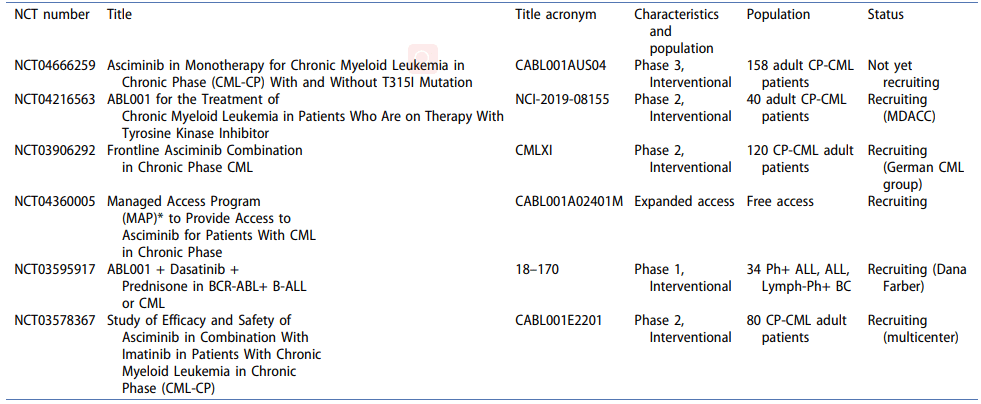
Clinical trials of Asciminib and its efficacy in treating CML
Clinical trials of Asciminib have shown promising results in the treatment of chronic myeloid leukemia (CML). In a phase 1 trial, Asciminib was well-tolerated and showed promising efficacy in patients with CML who were resistant to or intolerant of previous TKI therapy. In a subsequent phase 2 trial, patients with CML who were resistant to or intolerant of multiple TKIs showed significant response rates with Asciminib, including complete cytogenetic response and major molecular response. These results suggest that Asciminib may be an effective therapy for patients with refractory CML. Ongoing clinical trials will continue to evaluate the safety and efficacy of Asciminib in larger patient populations, with the goal of improving outcomes for patients with CML.
Advantages and Limitations of Asciminib
Asciminib has several advantages over traditional tyrosine kinase inhibitors (TKIs) in the treatment of chronic myeloid leukemia (CML). As an allosteric inhibitor, Asciminib targets a different site on the BCR-ABL protein than traditional TKIs, which can overcome resistance to TKIs in some patients. Additionally, Asciminib has shown promising results in clinical trials, including in patients who have failed multiple previous therapies.
However, there are also several limitations to the use of Asciminib that need to be considered. One limitation is the lack of long-term safety and efficacy data, as Asciminib is a relatively new therapy that has only been studied in a limited number of patients. Additionally, the cost of Asciminib may be a barrier to access for some patients, as newer therapies are often more expensive than traditional therapies. Finally, there is a risk of developing resistance to Asciminib over time, as is the case with other targeted therapies, which may limit its long-term effectiveness.
In summary, Asciminib represents a promising new therapy for the treatment of CML, with several potential advantages over existing therapies. However, further research is needed to fully evaluate its safety and efficacy, and to determine its optimal use in the management of CML.
Table 2. Summary of preliminary clinical activity of asciminib.[4]
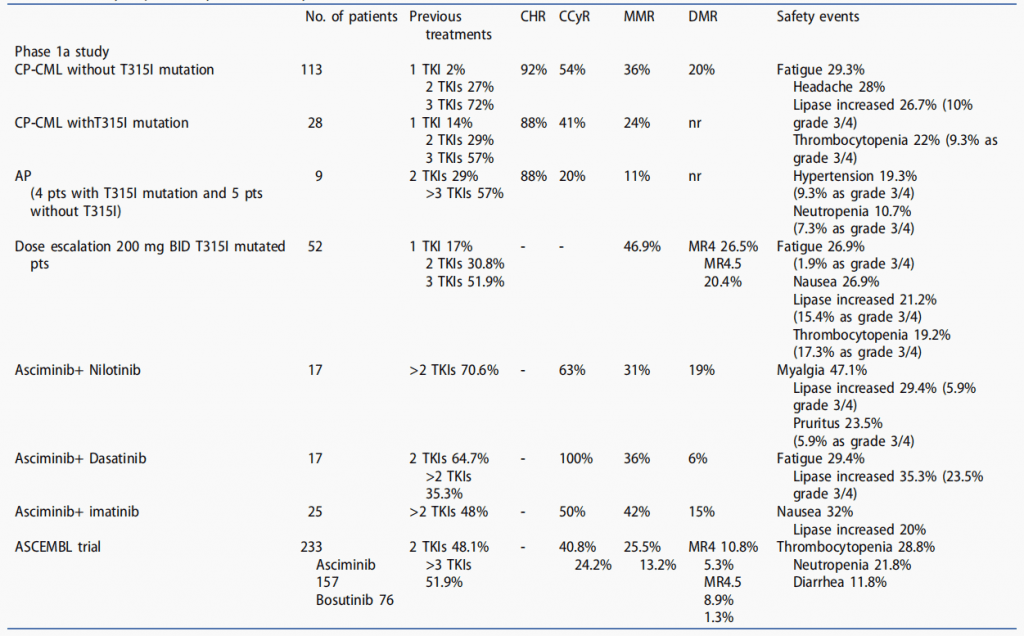
Future research directions for the development of Asciminib
Asciminib is a novel allosteric inhibitor of BCR-ABL1 that has shown promise as a new therapy for chronic myeloid leukemia (CML). The drug works by targeting a different site on the BCR-ABL1 protein than existing tyrosine kinase inhibitors (TKIs), which may make it effective against TKI-resistant CML. Preclinical studies have demonstrated that asciminib has potent anti-leukemic activity, with low toxicity and good tolerability.
Clinical trials have also shown promising results for asciminib, with high rates of hematologic and molecular responses in patients with CML who had previously failed treatment with multiple TKIs. However, some limitations have been identified, including a higher incidence of gastrointestinal side effects compared to other TKIs, and the need for twice-daily dosing.
Despite these limitations, asciminib represents a potential new treatment option for CML patients, particularly those who have become resistant to existing therapies. Further studies are needed to fully understand its long-term safety and efficacy, as well as its role in combination with other treatments.
Reference
- Wu, P., Clausen, M. H., & Nielsen, T. E. (2015). Allosteric small-molecule kinase inhibitors. Pharmacology & therapeutics, 156, 59-68.
- Réa, D., & Hughes, T. P. (2022). Development of asciminib, a novel allosteric inhibitor of BCR-ABL1. Critical Reviews in Oncology/Hematology, 103580.
- Nussinov, R., Zhang, M., Maloney, R., Liu, Y., Tsai, C. J., & Jang, H. (2022). Allostery: allosteric cancer drivers and innovative allosteric drugs. Journal of molecular biology, 167569.
- Breccia, M., Colafigli, G., Scalzulli, E., & Martelli, M. (2021). Asciminib: an investigational agent for the treatment of chronic myeloid leukemia. Expert Opinion on Investigational Drugs, 30(8), 803-811.
- Attwood, M. M., Fabbro, D., Sokolov, A. V., Knapp, S., & Schiöth, H. B. (2021). Trends in kinase drug discovery: Targets, indications and inhibitor design. Nature Reviews Drug Discovery, 20(11), 839-861.
- Deeks, E. D. (2022). Asciminib: first approval. Drugs, 82(2), 219-226.