Antibody Drug Conjugates: A Comprehensive Guide
Abstract
Antibody-drug conjugates (ADCs) represent a potent fusion of chemotherapy and immunotherapy. The genesis of this concept traces back over a century to the visionary ideas of Paul Ehrlich, a German scientist. Ehrlich’s analogy likens ADCs to “enchanted medicines,” adeptly pinpointing specific targets like cancer cells, analogous to skilled “sharpshooters.” In contemporary times, the escalating endorsement and commercialization of ADCs have propelled this genre into a prominent domain within the realm of global biotechnological drug exploration and advancement. This narrative delves into the remarkable attributes of ADCs and dissects the mechanism underlying their remarkable “precision guidance.” Noteworthy inquiries about ADCs, encompassing:
- What is an antibody-drug conjugate?
- Antibody-drug conjugate structure
- How do antibody-drug conjugates work?
- Antibody-drug conjugate composition
- Advantages of antibody-drug conjugates
- Are there any weaknesses of antibody-drug conjugates?
- How many FDA-approved ADCs are there?
1. What is an antibody-drug conjugate?
An Antibody-drug conjugate (ADC) emerges as an innovative class of biological medication, seamlessly melding the pinpoint accuracy characteristic of monoclonal antibody drugs with the potent efficacy exhibited by small molecule cytotoxic drugs. This union enhances the precision targeting of therapeutic agents for tumors while concurrently mitigating the impact of toxic adverse effects.
2. Antibody-drug conjugate structure
The ADC comprises three primary components: an antibody tasked with discerning antigens exclusive to cancer cell surfaces, a payload entrusted with eradicating cancer cells, and a linker that facilitates the connection between the antibody and the payload.
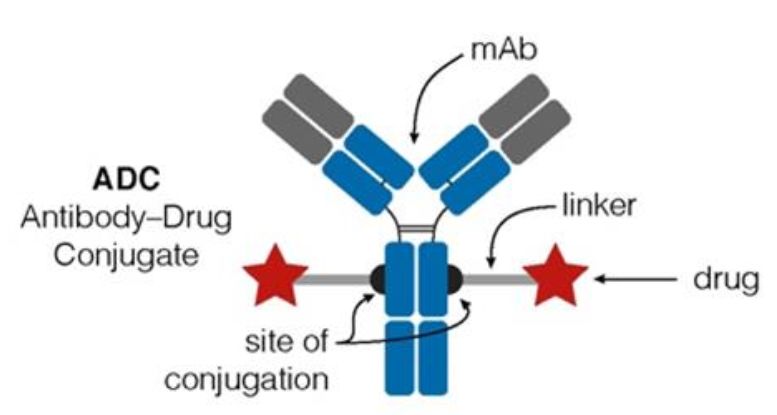
Figure 1. Structure of ADC drug
3. How do antibody-drug conjugates work?
Initially, following its introduction into the body, the ADC binds to the target cell antigen after traversing numerous barriers. This interaction culminates in the creation of an ADC-antigen complex that gains entry into the cell via plectin-facilitated endocytosis. Once inside the cell, the complex experiences fusion between the lysosome and endosome, leading to the cleavage of the intracellular linker. This action results in the liberation and activation of the small-molecule cytotoxic drug. Upon release into the cytoplasm, the drug exerts its effects, which encompass DNA insertion or the inhibition of microtubule polymerization. Consequently, tumor cells are eliminated, prompting apoptosis in the targeted cells. Notably, the potent payload’s impact may extend to neighboring tumor cells upon the demise of the target cell, a phenomenon known as the bystander effect.
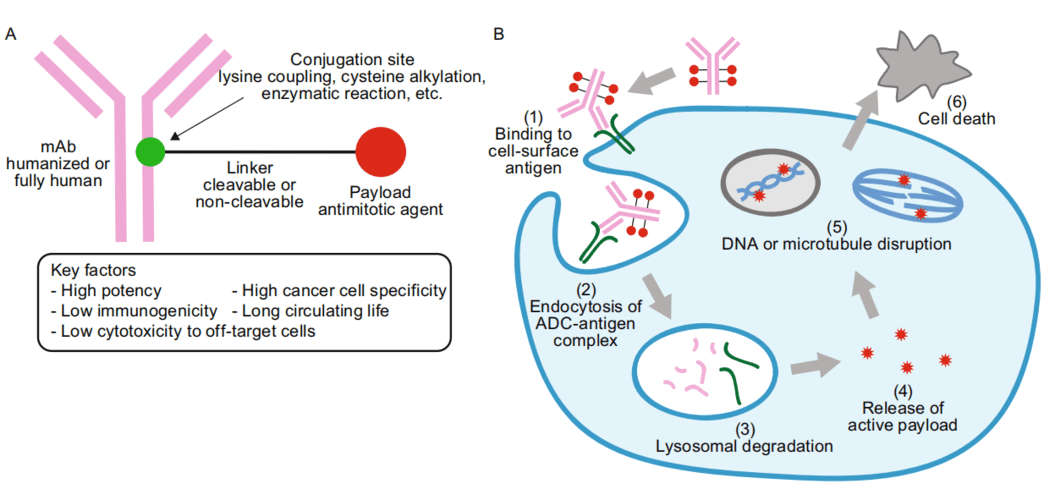
Figure 2. ADC drug working mechanism
4. Antibody-drug conjugate composition
4.1 Selection of target
The triumphant advancement of ADCs hinges on the precise bonding of the antibody with its designated target. The prime objective for an ADC is for the target to exhibit robust expression exclusively on the tumor cell surface while registering minimal to no presence in normal cells. This duality aims to curtail the likelihood of both focused and unintended toxicity toward tumor cells. Beyond specificity and substantial expression, the ultimate target should also trigger effective internalization responses.
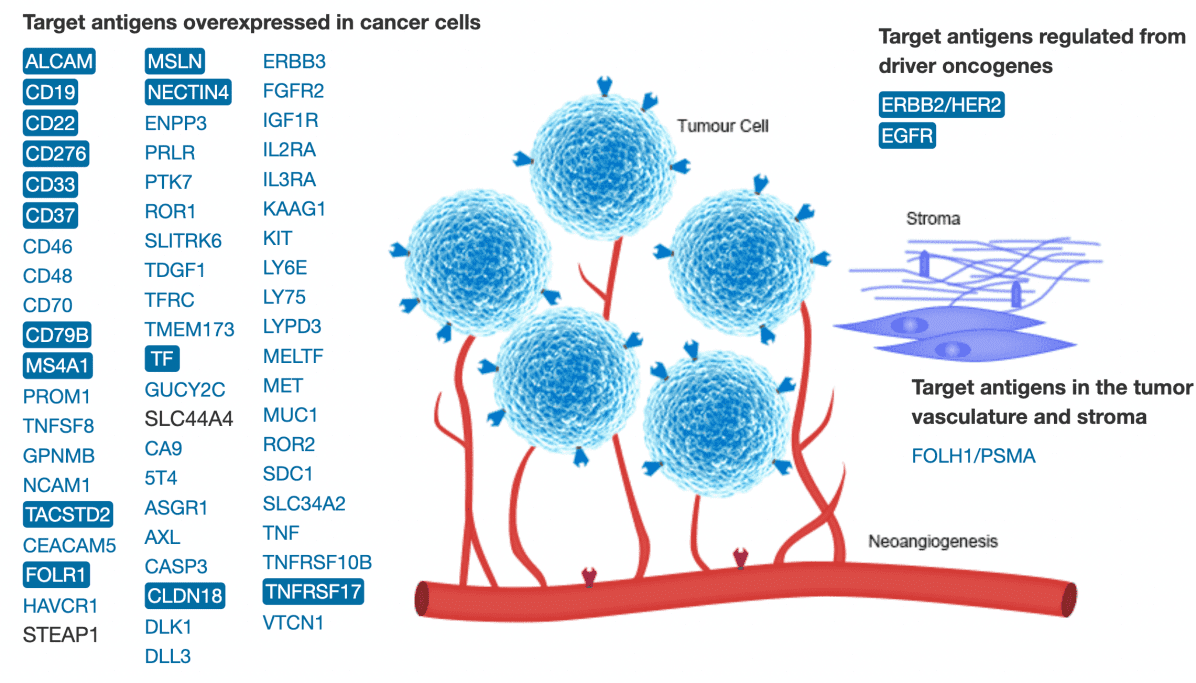
Figure 3. Targets in ADC drug development (including drugs under development, clinical, and on the market) (A). Targeting antigens overexpressed in cancer cells; (B). Antigens regulated by oncogenes; (C). Target antigens in tumor-bearing blood vessels and stroma
4.2 Selection of Antibody
Antibodies necessitate elevated antigen affinity and an extended circulating half-life to effectively concentrate the cytotoxin at the tumor locus. When evaluating characteristics such as half-life, structural resilience, immune function of the Fc fragment, and ease of conjugation among diverse IgG antibody variants, ADCs predominantly opt for IgG1, while IgG4 is seldom employed. Categorizing antibodies based on immunogenicity, they fall into fully humanized, humanized, and chimeric types. To minimize immune reactions, a preference is given to selecting more fully humanized or humanized antibodies.
4.3 Selection of payload
The cytotoxic payload molecule plays a pivotal role in ensuring the triumph of ADC advancement. Given the sequence of events that the ADC must traverse – from its introduction into the human body to the eventual discharge of the cytotoxic entity – meticulous consideration of each step’s efficiency is paramount. Consequently, the payload should exhibit robust anti-tumor potency, rendering toxic molecules with efficacy at the nanomolar scale (characterized by IC50 values ranging from 0.01 to 0.1 nM) well-suited for this role. Furthermore, the payload must possess compatible functional groups for seamless coupling, display potent cytotoxic attributes, strike an appropriate hydrophilic-hydrophobic equilibrium, and exhibit commendable stability.
4.4 Selection of linkers
The linker serves as a conduit, establishing a connection between the ADC and the antibody via either a cleavable or non-cleavable linker. The construction of this bridge necessitates meticulous design, incorporating stability to resist disintegration under physiological circumstances, while concurrently exhibiting traits conducive to proficient release at designated locations.
5. Advantages of antibody-drug conjugates
In contrast to conventional fully or partially humanized antibodies or antibody fragments, ADCs offer the capacity to liberate profoundly potent cytotoxins within tumor tissues, potentially yielding elevated efficacy. In parallel, ADCs exhibit superior tolerability and diminished adverse effects compared to fusion proteins. Their ability to precisely discriminate targets without impacting healthy cells significantly enhances therapeutic outcomes and minimizes detrimental effects, thereby garnering substantial interest from professionals engaged in pharmaceutical research and development.
6. Are there any weaknesses of antibody-drug conjugates?
ADCs exhibit intricate structural complexity and a wide array of design variations, introducing challenges to the manufacturing and quality control aspects of CMC research. Simultaneously, the intricate overlay of biological processes within ADCs in vivo presents manifold hurdles for both non-clinical and clinical investigations. Given that ADC production predominantly entails the utilization of exceptionally potent cytotoxic agents, it demands stringent prerequisites concerning hardware, process blueprint, and personnel expertise. This mandates substantial capital investment and substantial technical preparedness, consequently raising the bar for production entry.
Currently, ADC development faces three major challenges and opportunities:
6.1 Linker instability
This instability can lead to premature release of the payload into the blood and lead to nonspecific uptake of ADCs and on-target toxicity.
6.2 Nonspecific endocytosis
Increased hydrophobicity can drive the aggregation and indiscriminate endocytosis of ADCs, particularly those with elevated DAR (drug-to-antibody ratio), ultimately leading to both targeted and non-targeted effects. Additionally, ADCs featuring high DAR are prone to clearance by non-specific endocytic cells. Consequently, optimizing the DAR presents a significant tactic for enhancing the therapeutic index (TI).
6.3 Receptor-mediated uptake mechanism
Hematotoxicity predominantly represents the off-target toxicity of ADC facilitated through Fc γ receptors (FcγRs). Among ADCs incorporating Auristatin (MAME, MMAF), Calicheamicin, and Maytansinoid (DM-1), hematological toxicity emerged as the primary off-target dose-limiting toxicity (DLT).
7. FDA-approved antibody-drug conjugates
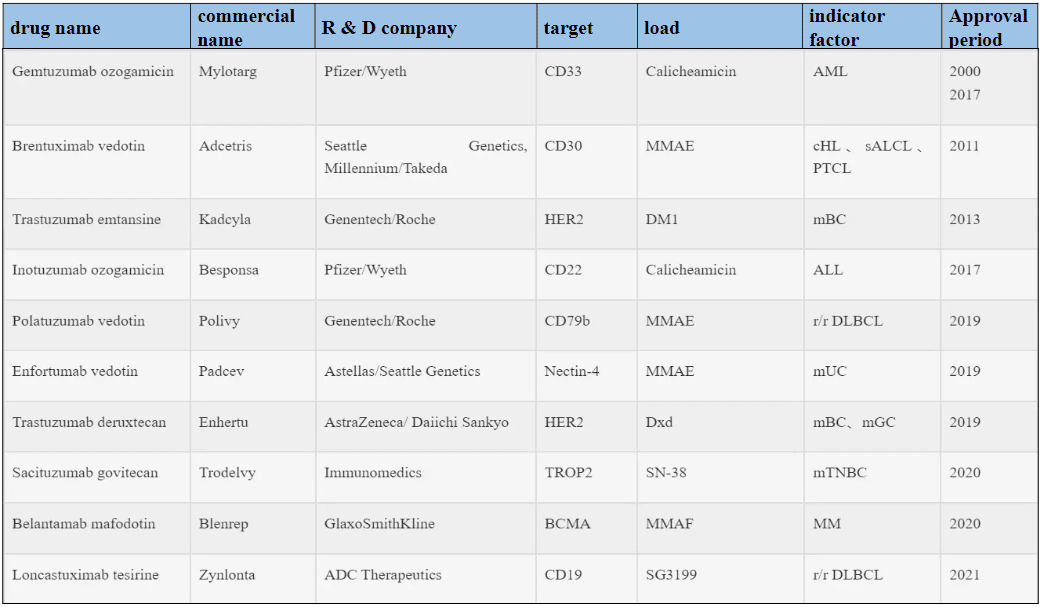
Among the 10 ADCs granted marketing approval, six are designated for treating hematological malignancies, while the remaining four are targeted at solid tumors (Refer to Table 1). Presently, over 80 ADCs are actively undergoing clinical trials, predominantly situated within phases I and I/II. Notably, more than 80% of these clinical investigations are dedicated to evaluating the safety and effectiveness of ADCs within diverse solid tumor contexts, with the remainder addressing hematological malignancies. This discernible trend highlights the gradual pivot towards solid tumor research in the ADC field, fueled by the early triumph of T-DM1 and the recent endorsements of sacituzumab govitecan and Loncastuximab tesirine.
Table 1. FDA-approved ADC drugs
Ever since the concept of the “biological missile” was introduced, the realm of ADC has experienced ongoing innovation and optimization, leading to significant advancements. This has positioned ADC as a crucial modality within the sphere of cancer treatment. As a burgeoning number of ADCs transition into the clinical stage, the industry is progressively veering away from conventional techniques, embracing more pioneering methodologies for the development of these intricate products. This includes the exploration of novel tumor antigens, inventive antibody architectures, fresh payload options, innovative linker designs, and advanced conjugation techniques. As ADC research deepens, the molecular blueprint of ADCs is expected to become more rational, yielding enhanced uniformity and bolstered in vivo stability. This trajectory aims to diminish toxicities and side effects, amplify efficacy and potency, widen the therapeutic margin, and ultimately introduce newfound hope through novel medications for cancer patients.
References
- Abuhelwa Z, Alloghbi A, Nagasaka M. A comprehensive review on antibody-drug conjugates (ADCs) in the treatment landscape of non-small cell lung cancer (NSCLC)[J]. Cancer treatment reviews, 2022, 106.
- Nguyen TD, Bordeau BM, Balthasar JP. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers (Basel). 2023 Jan 24;15(3):713.
- Hammood M, Craig AW, Leyton JV. Impact of Endocytosis Mechanisms for the Receptors Targeted by the Currently Approved Antibody-Drug Conjugates (ADCs)-A Necessity for Future ADC Research and Development. Pharmaceuticals (Basel). 2021 Jul 15;14(7):674.
- Goldenberg DM, Sharkey RM. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther. 2020 Aug;20(8):871-885.
- Baah S, Laws M, Rahman KM. Antibody-Drug Conjugates-A Tutorial Review. Molecules. 2021 May 15;26(10):2943.
- Joubert N, Beck A, Dumontet C, Denevault-Sabourin C. Antibody-Drug Conjugates: The Last Decade. Pharmaceuticals (Basel). 2020 Sep 14;13(9):245.
- Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-Drug Conjugates: A Comprehensive Review. Mol Cancer Res. 2020 Jan;18(1):3-19.