Analysis of the mechanism of action of ADC drugs
Abstract
Antibody-Drug Conjugates (ADCs) are a class of targeted cancer therapies combining monoclonal antibodies with cytotoxic drugs via stable linkers. The antibody component selectively binds to tumor-specific antigens, enabling the delivery of highly potent toxins directly to cancer cells. Once internalized, the linker is cleaved (chemically or enzymatically), releasing the cytotoxic agent that induces apoptosis. Some ADCs exert a bystander effect, allowing the drug to kill neighboring tumor cells, influenced by linker stability and payload polarity. Additionally, ADCs can stimulate anti-tumor immune responses through mechanisms like ADCC, CDC, and ADCP. Their precision and efficacy make ADCs a powerful and growing option in oncology.
Introduction
Antibody-Drug Conjugates (ADC) are made of monoclonal antibodies (Antibody) targeting tumor-specific antigens or tumor-associated antigens, coupled with different numbers of small molecule cytotoxins (Payload) through linkers. They have the dual advantages of high targeting of monoclonal antibodies and high activity of cytotoxic drugs in tumor tissues, and are one of the fastest-growing drug categories in the field of tumor targeted therapy in recent years.
Components of ADC drugs
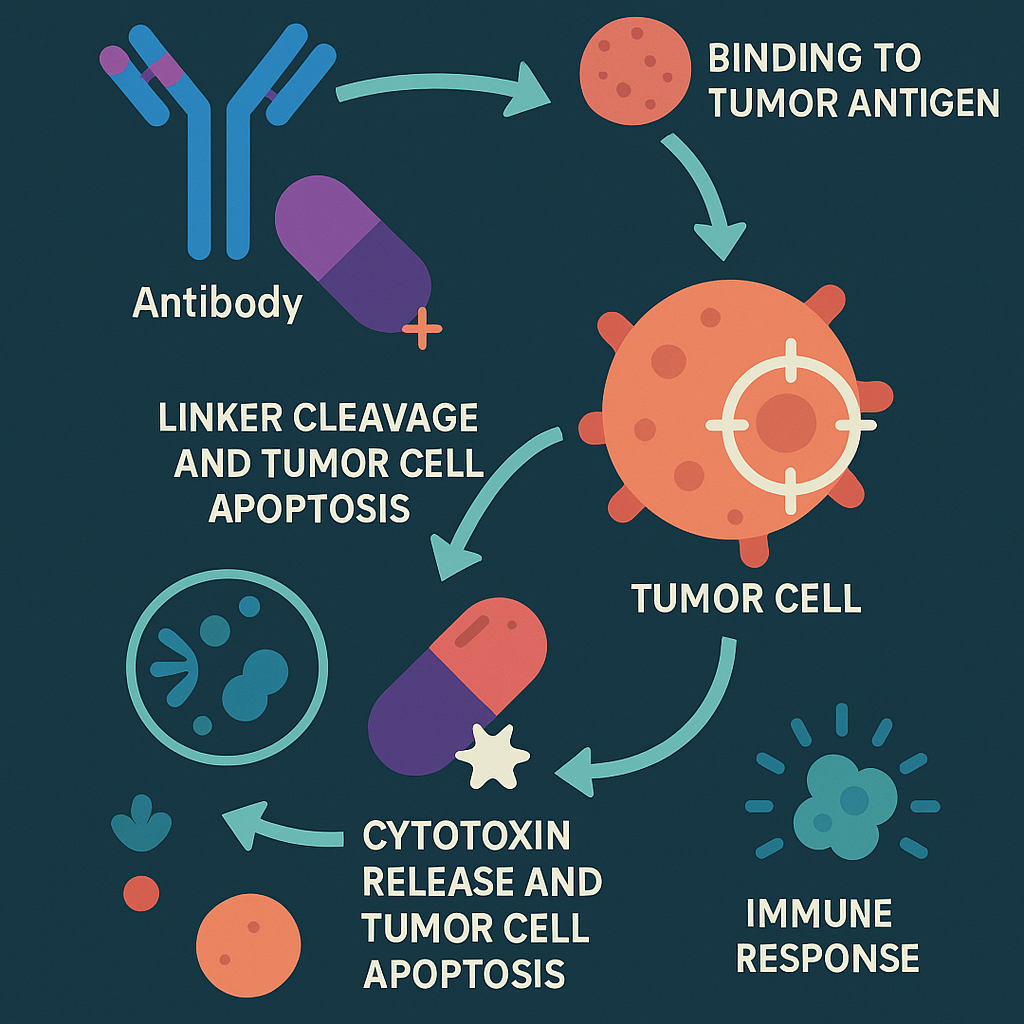
1) [Navigation system] Monoclonal antibody (Antibody): Monoclonal antibody designed for specific target antigens on the surface of tumor cells [precisely binds like a key to open a lock];
2) [Stable chain] Linker: It can stably connect the toxin and antibody by covalent bond [ensuring that the drug does not “go off target” before reaching the tumor and is safely transported];
3) [Warhead] Cytotoxic small molecule (Cytotoxic drug): Highly active cytotoxin, that is, chemotherapy drug [“ammunition” that efficiently kills tumor cells, with a power far exceeding that of ordinary chemotherapy].
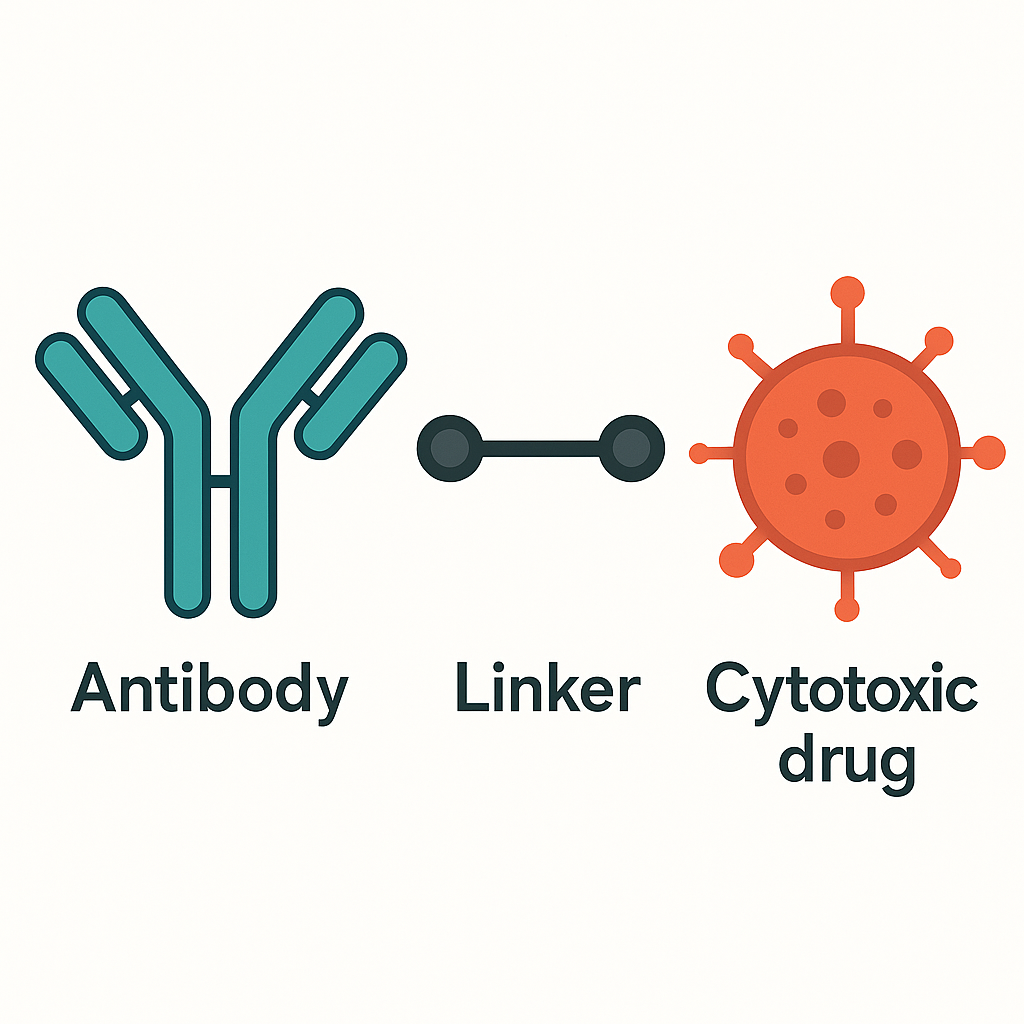
Ideal ADC drugs remain stable in the blood circulation, accurately reach the therapeutic target, and eventually release cytotoxic payloads near the target (such as cancer cells).
Mechanism of action of ADC drugs
In the past decade, antibody-drug conjugates (ADCs) have become a treatment for a variety of solid and hematological malignancies. ADCs synergistically play the role of “specific” targeting and “efficient” killing of cancer cells. This type of drug is like a precision-guided “biological missile” that can accurately destroy cancer cells, increase the treatment window, and reduce off-target side effects.
Main mechanism of action
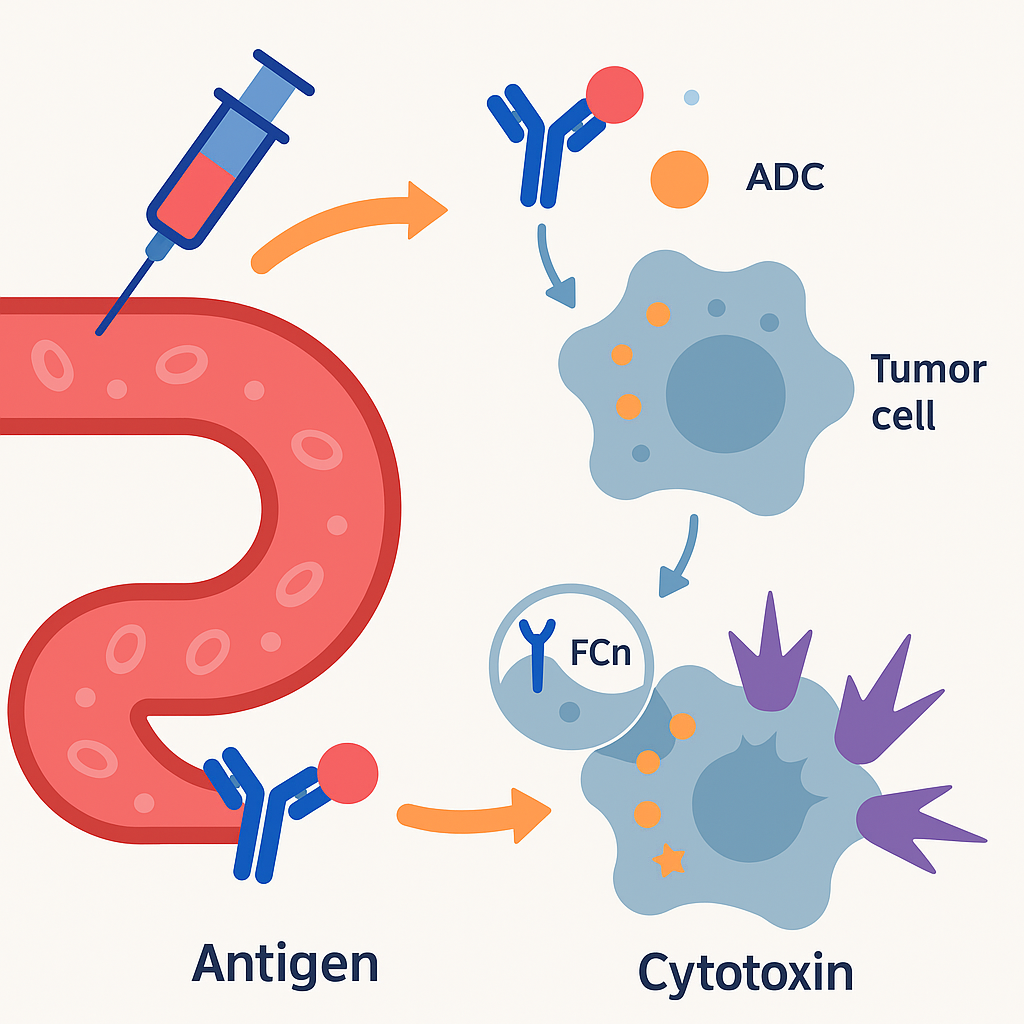
After intravenous injection, ADC drugs enter the blood circulation and bind to the target antigen receptors on the surface of tumor cells to form ADC-antigen complexes. Under receptor-mediated endocytosis, some ADC-antigen complexes will bind to the FcRn receptors in the endosomes, be released back to the extracellular space, and re-enter the blood circulation. This is a buffer mechanism for normal cells to swallow by mistake. Some ADC-antigen complexes will be hydrolyzed by lysosomes, and after the linker breaks, active cytotoxins will be released. The released active cytotoxicity begins to interfere with key cell mechanisms, leading to cell apoptosis.
Bystander effect
Bystander cells are actually cells around tumor cells, including heterogeneous tumor cells and the above-mentioned metastatic tumor cells. These nearby cancer cells can be Ag+ or Ag-. The bystander effect is that the drug can also kill these cells. Not all ADCs have this effect, which is often related to the biochemical properties of the linker and cytotoxin.
The bystander effect can kill cells around the target tumor cells, which is very different from ADCs without this effect. There are many factors that affect the bystander effect.
First, the stability of the chemical bond between the antibody and the linker is one of the influencing factors. ADCs without the bystander effect will not release cytotoxins before reaching tumor cells. Therefore, the chemical bond is very stable and will not break easily. ADCs with relatively weak stability will lead to the premature release of cytotoxins, which have the opportunity to reach cells near tumor cells and produce a bystander effect.
Secondly, the stability of the linker is the same, which is also one of the factors leading to the bystander effect. Linkers are usually divided into cleavable and non-cleavable. The former includes chemically unstable connections (such as pH-sensitive hydrazone bonds and disulfide bonds) and enzyme-unstable connections (such as dipeptides, which can be cleaved by histone B). The latter is mainly covalently linked (such as thioether bonds), which must be internalized into cells and decomposed by lysosomes to release toxins.
The nature of cytotoxins is also an important factor affecting the bystander effect. Toxins with low polarity can easily penetrate the cell membrane. Therefore, after being released in tumor cells, they may still diffuse through the cell membrane to the outside of the tumor, thereby acting on surrounding cells and producing a bystander effect. Toxins with too high polarity are difficult to penetrate the cell membrane. There are also special cases here. For example, although the polarity of T-DM1 is not very large, due to its incomplete cleavage, a positively charged lysine remains on the toxin, which makes it unable to penetrate the cell membrane. Therefore, T-DM1 will not produce a bystander effect.
In addition, when the target tumor cells finally disintegrate, the cytotoxins inside them will be released and diffuse to the surrounding cells, exerting a bystander effect.
Anti-tumor immunity and cytokine activation pathways
The antibody component of ADC drugs interacts with immune effector cells to induce anti-tumor immunity, including complement-dependent cytotoxicity (CDC), antibody-dependent cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP). The antibody Fab fragments of some ADCs can bind to antigenic epitopes of virus-infected cells or tumor cells, while the FC fragments bind to FCR on the surface of killer cells (NK cells, macrophages, etc.), thereby mediating direct killing effects. The antibodies of some ADC drugs can maintain their activity and still have the function of interfering with the target, inhibiting downstream signaling pathways, and thus inhibiting tumor growth.

Conclusion
In conclusion, Antibody-Drug Conjugates (ADCs) represent a significant advancement in targeted cancer therapy by integrating the high specificity of monoclonal antibodies with the potent cytotoxic effects of chemotherapy drugs. Through precise targeting of tumor-associated antigens and controlled intracellular drug release, ADCs maximize therapeutic efficacy while minimizing systemic toxicity. The bystander effect further enhances their ability to eliminate heterogeneous tumor populations. Advances in linker chemistry, payload design, and antibody engineering continue to improve ADC safety, stability, and immune activation potential. As research evolves, ADCs are poised to become a cornerstone in the personalized treatment of both solid and hematologic malignancies, offering hope for more effective and less harmful cancer therapies.
References
Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022 Mar 22;7(1):93. doi: 10.1038/s41392-022-00947-7. PMID: 35318309; PMCID: PMC8941077.