AMPK and Autophagy: Unlocking the Cell’s Self-Cleaning Switch for Metabolic Health and Longevity
Abstract
AMP-activated protein kinase (AMPK) is a central energy sensor that plays a pivotal role in maintaining cellular homeostasis, especially during metabolic stress. One of its most critical functions is the regulation of autophagy—a self-degradative process essential for clearing damaged organelles, misfolded proteins, and other cellular debris. This blog explores the structure and activation mechanisms of AMPK, its role in initiating and controlling autophagy, and its broader implications in metabolic diseases, neurodegeneration, and cancer. With growing evidence supporting AMPK as a therapeutic target, enhancing this pathway offers promising strategies for promoting longevity, improving energy metabolism, and restoring cellular resilience.
AMPK and Autophagy: The Cellular Duo Reshaping Metabolic Health
In the dynamic environment of the human body, cellular survival depends on one core principle: energy balance. Every biological function, from muscle contraction to memory formation, relies on the efficient management of cellular energy. When energy levels dip—whether due to nutrient deprivation, oxidative stress, or aging—cells activate survival pathways to adapt. Two of the most critical players in this adaptive response are AMPK (AMP-activated protein kinase) and autophagy, forming a powerful partnership that is now being recognized as central to metabolic health.
AMPK is often called the cell’s “fuel gauge.” It senses shifts in the AMP/ATP ratio and responds to low energy by halting energy-consuming processes while activating pathways that generate ATP. This kinase doesn’t just respond passively; it coordinates a complex cellular program to restore homeostasis. One of its most significant downstream effects is the activation of autophagy, the cell’s built-in recycling system.
Autophagy, meaning “self-eating,” is a process by which cells degrade and reuse damaged organelles, misfolded proteins, and invasive pathogens. It prevents cellular clutter and contributes to rejuvenation, stress resistance, and metabolic adaptability. Importantly, autophagy isn’t random—it’s carefully regulated, and AMPK is one of its most powerful switches.
When AMPK is activated under stress, it triggers autophagy to clear out unnecessary or damaged cellular components, thus restoring internal balance and improving energy efficiency. This process is vital not only in normal physiology but also in the context of aging, cancer, cardiovascular disease, neurodegeneration, and type 2 diabetes. The AMPK-autophagy axis has therefore emerged as a critical target for metabolic therapies, especially those seeking to promote longevity, reduce inflammation, and restore cellular function.
Understanding how AMPK controls autophagy helps us appreciate the cell’s innate intelligence in handling stress. It also opens the door to new therapeutic strategies—many of which are already being explored through exercise, caloric restriction, and pharmacological activators like metformin and resveratrol. As we’ll explore in this blog series, the AMPK-autophagy pathway is much more than a molecular detail—it’s a cornerstone of cellular life.
Meet AMPK — The Energy Sensor Enzyme
At the heart of every metabolic adjustment within the cell lies a sophisticated enzyme that acts like an energy thermostat: AMP-activated protein kinase (AMPK). As cells constantly toggle between energy surplus and deficit, AMPK monitors intracellular energy status and ensures a precise response to restore balance. Its critical role in maintaining cellular and whole-body energy homeostasis has earned it a central position in metabolic research.
AMPK is a serine/threonine kinase found in all eukaryotic cells. It is activated when cellular energy levels fall, such as during exercise, fasting, hypoxia, or glucose deprivation. These conditions elevate the AMP/ATP or ADP/ATP ratio, signaling a drop in available energy. In response, AMPK becomes activated and shifts the cell’s metabolism toward ATP generation while inhibiting energy-consuming processes like lipid synthesis, protein synthesis, and cell growth.
Structurally, AMPK is a heterotrimeric complex composed of three subunits:
The α-subunit contains the catalytic domain, including the critical Thr172 site for activation.
The β-subunit serves as a scaffold and contains a carbohydrate-binding module (CBM) that helps localize AMPK to glycogen stores.
The γ-subunit houses four CBS (cystathionine β-synthase) domains, which bind adenine nucleotides (AMP, ADP, ATP) to sense the energy state of the cell.
Activation of AMPK can occur through three primary mechanisms:
Allosteric activation by AMP binding to the γ-subunit.
Phosphorylation at Thr172 by upstream kinases such as LKB1, CaMKKβ, or TAK1.
Inhibition of dephosphorylation, whereby AMP binding protects Thr172 from being deactivated by phosphatases.
Once activated, AMPK inhibits anabolic pathways and enhances catabolic processes, including fatty acid oxidation, glycolysis, mitochondrial biogenesis, and notably, autophagy. This adaptive switch helps cells conserve energy and survive under metabolic stress.
By acting as a master regulator of energy metabolism, AMPK not only coordinates short-term survival but also plays a preventive role in chronic diseases such as obesity, cancer, and type 2 diabetes. Understanding how AMPK senses and regulates energy provides valuable insight into therapeutic strategies aimed at restoring metabolic health.
AMPK as the Master Switch of Autophagy
Autophagy is more than just cellular housekeeping—it’s a sophisticated survival mechanism tightly controlled by nutrient availability and energy status. At the helm of this regulation is AMPK (AMP-activated protein kinase), which serves as a critical activator of autophagy, especially under conditions of metabolic stress. When energy levels drop, AMPK initiates a cascade of events that ignite autophagy from the ground up, positioning it as the master switch for autophagic induction.
The initiation of autophagy begins with the activation of ULK1 (Unc-51-like kinase 1), a key regulator of autophagosome formation. AMPK directly phosphorylates ULK1 at several essential residues—Ser467, Ser555, Thr574, and Ser637—boosting its activity. This activation recruits core autophagy proteins such as ATG13, FIP200, and ATG101 to the phagophore formation site, where the isolation membrane begins to develop.
Simultaneously, AMPK inhibits one of the strongest suppressors of autophagy: the mTORC1 (mechanistic target of rapamycin complex 1). Under nutrient-rich conditions, mTORC1 blocks autophagy by suppressing ULK1 and ATG13. However, AMPK reverses this inhibition in two powerful ways:
It phosphorylates RAPTOR (a regulatory subunit of mTORC1) on Ser722 and Ser792, suppressing mTORC1 activity.
It activates the TSC1/TSC2 complex, which further inhibits mTORC1 by blocking Rheb, a small GTPase required for mTORC1 function.
By antagonizing mTORC1 and activating ULK1, AMPK orchestrates a dual mechanism that ensures autophagy is promptly initiated during energy stress. This tightly coordinated regulation allows the cell to divert its resources away from growth and toward survival, removing damaged components and generating energy through recycling.
Moreover, AMPK enhances autophagic signaling by influencing Class III PI3K complexes, particularly the VPS34–Beclin1–ATG14 complex, which promotes autophagosome nucleation. This reinforces the pro-autophagic environment at multiple levels.
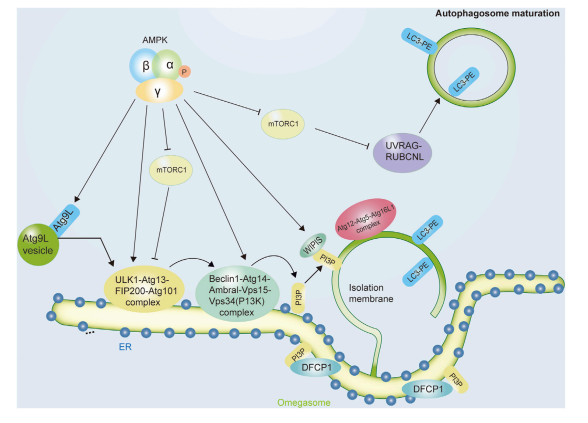
FIGURE 1. The role of AMPK in initiating the autophagic process. A
In essence, AMPK does not merely support autophagy—it initiates and governs it, acting as a metabolic conductor that cues cells when it’s time to clean house and adapt. This function is pivotal in conditions ranging from cancer to aging, making AMPK a focal point for therapeutic interventions aimed at modulating autophagy.
Building and Completing Autophagosomes: AMPK’s Role in Autophagic Progression
Once autophagy is initiated, the next challenge for the cell is to build and complete the autophagosome, a double-membraned vesicle that sequesters damaged organelles, protein aggregates, and other cellular debris. This process, known as autophagosome biogenesis, is tightly regulated and relies heavily on the orchestrating activity of AMPK. Beyond just triggering autophagy, AMPK plays an integral role in ensuring that autophagosomes are properly formed, matured, and ultimately fused with lysosomes for degradation.
During biogenesis, AMPK facilitates the expansion of the phagophore, the membrane precursor of the autophagosome. It supports the activity and assembly of the Class III PI3K complex, which includes VPS34, Beclin1, ATG14, and AMBRA1. AMPK directly phosphorylates Beclin1 at Thr388, enhancing its binding to VPS34 and boosting PI3K activity. This phosphorylation increases the recruitment of autophagy-related proteins and promotes vesicle nucleation and membrane expansion.
AMPK also fine-tunes the balance between different VPS34-containing complexes, enhancing those with pro-autophagic roles while suppressing others involved in unrelated cellular processes. For instance, it phosphorylates VPS34 at Thr163 and Ser165 to inhibit its activity in non-autophagic contexts, ensuring that cellular resources are directed exclusively toward autophagy under energy stress.
Another critical function of AMPK is to assist in the fusion of autophagosomes with lysosomes, a step that forms the autolysosome, where degradation occurs. This fusion requires coordination between vesicle positioning and membrane dynamics. AMPK indirectly promotes this step through the activation of ATG14, a scaffold protein that supports the SNARE complex involved in membrane fusion. Moreover, AMPK activation enhances lysosomal biogenesis through transcriptional regulation involving the SIRT1-TFEB axis, boosting the cell’s degradative capacity.
This stage of autophagy is not merely mechanical—it’s a refined, energy-sensitive process that determines the efficiency of cellular detoxification. Disruptions here can lead to the accumulation of autophagosomes and impaired degradation, contributing to neurodegenerative diseases and other pathologies. AMPK ensures that autophagy runs to completion, maintaining cellular health through tight energy and resource control.
Clinical Insights and Therapeutic Potential of AMPK-Driven Autophagy
As research into cellular energy metabolism advances, AMPK has emerged not just as a biological regulator but as a promising therapeutic target. Its central role in activating autophagy connects metabolic stress responses with disease prevention and recovery. By enhancing autophagic flux, AMPK helps cells remove dysfunctional components, reduce oxidative stress, and recycle valuable biomolecules—processes that are impaired in many chronic diseases.
In metabolic disorders such as type 2 diabetes and obesity, impaired autophagy contributes to insulin resistance and lipid accumulation. AMPK activation reverses these effects by promoting lipid oxidation and autophagic clearance of lipid droplets (lipophagy), restoring metabolic balance. The widely used anti-diabetic drug metformin is known to exert some of its effects by activating AMPK, indirectly stimulating autophagy and improving insulin sensitivity.
In neurodegenerative diseases like Alzheimer’s, Parkinson’s, and Huntington’s disease, protein aggregates and damaged mitochondria accumulate due to defective autophagy. Activating AMPK helps mitigate this by enhancing mitophagy (mitochondrial autophagy) and clearing neurotoxic waste. Natural compounds like resveratrol, as well as exercise-induced AMPK activation, have shown neuroprotective effects through enhanced autophagy pathways.
Cancer presents a more complex relationship with autophagy and AMPK. While autophagy can support tumor survival under metabolic stress, AMPK also acts as a tumor suppressor by inhibiting anabolic growth signals via mTORC1 suppression. Early in cancer development, AMPK-driven autophagy helps maintain genomic stability and prevent tumorigenesis. Therapeutic strategies are now exploring AMPK activators to suppress tumor growth and enhance the efficacy of chemotherapy.
Emerging evidence also links AMPK-autophagy signaling to cardiovascular health, immune function, and longevity. Exercise, fasting, and caloric restriction—all of which activate AMPK—have shown widespread benefits on these systems, suggesting that enhancing this pathway may serve as a universal strategy to improve healthspan.
The therapeutic promise of AMPK lies in its ability to finely tune autophagy without overwhelming the system. Rather than acting as a blunt tool, AMPK activation mimics natural cellular responses, making it a biologically intelligent approach to disease prevention and treatment. As we continue to unlock its mechanisms, AMPK stands as a powerful link between metabolism, cellular cleanup, and resilience.
References
Bonanno, L., Zulato, E., Pavan, A., Attili, I., Pasello, G., Conte, P., et al. (2019). LKB1 and tumor metabolism: The interplay of immune and angiogenic microenvironment in lung cancer. International Journal of Molecular Sciences, 20(8), 1874.
https://doi.org/10.3390/ijms20081874
Hardie, D. G., Ross, F. A., & Hawley, S. A. (2012). AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology, 13(4), 251–262.
https://doi.org/10.1038/nrm3311
Mihaylova, M. M., & Shaw, R. J. (2011). The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biology, 13(9), 1016–1023.
https://doi.org/10.1038/ncb2329
Zhao, B., Qiang, L., Joseph, J., Kalyanaraman, B., Viollet, B., & He, Y. Y. (2016). Mitochondrial dysfunction activates the AMPK signaling and autophagy to promote cell survival. Genes & Diseases, 3(1), 82–87.
https://doi.org/10.1016/j.gendis.2015.12.002
Carling, D. (2017). AMPK signalling in health and disease. Current Opinion in Cell Biology, 45, 31–37.
https://doi.org/10.1016/j.ceb.2017.01.005
Hardie, D. G., Schaffer, B. E., & Brunet, A. (2016). AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends in Cell Biology, 26(3), 190–201.
https://doi.org/10.1016/j.tcb.2015.10.013
Herzig, S., & Shaw, R. J. (2018). AMPK: Guardian of metabolism and mitochondrial homeostasis. Nature Reviews Molecular Cell Biology, 19(2), 121–135.
https://doi.org/10.1038/nrm.2017.95
Jeon, S. M. (2016). Regulation and function of AMPK in physiology and diseases. Experimental & Molecular Medicine, 48(7), e245.
https://doi.org/10.1038/emm.2016.81
Egan, D., Kim, J., Shaw, R. J., & Guan, K. L. (2011). The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy, 7(6), 643–644.
https://doi.org/10.4161/auto.7.6.15123
Huang, Y., Li, S., Jia, Z., Zhao, W., Zhou, C., Zhang, R., et al. (2020). TRPM8 channel regulates proliferation and migration of breast cancer cells by activating the AMPK-ULK1 pathway to enhance basal autophagy. Frontiers in Oncology, 10, 573127.
https://doi.org/10.3389/fonc.2020.573127
Kim, J., Kundu, M., Viollet, B., & Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology, 13(2), 132–141.
https://doi.org/10.1038/ncb2152
Rabinowitz, J. D., & White, E. (2010). Autophagy and metabolism. Science, 330(6009), 1344–1348.
https://doi.org/10.1126/science.1193497
Kim, J., Kim, Y. C., Fang, C., Russell, R. C., Kim, J. H., Fan, W., et al. (2013). Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell, 152(1–2), 290–303.
https://doi.org/10.1016/j.cell.2012.12.016
Russell, R. C., Tian, Y., Yuan, H., Park, H. W., Chang, Y. Y., Kim, J., et al. (2013). ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nature Cell Biology, 15(7), 741–750.
https://doi.org/10.1038/ncb2757
Moors, T., Hoozemans, J., Ingrassia, A., Beccari, T., Parnetti, L., Chartier-Harlin, M., et al. (2017). Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Molecular Neurodegeneration, 12(1), 11.




