AMPK Activators: Unlocking the Master Metabolic Switch for Health and Disease
Abstract
AMP-activated protein kinase (AMPK) serves as a central regulator of cellular energy homeostasis, coordinating metabolic pathways that balance ATP production and consumption. Recent research highlights the therapeutic potential of AMPK activators, ranging from clinically established drugs like metformin to natural polyphenols and novel synthetic molecules. These activators function through both indirect mechanisms, such as altering mitochondrial energy balance, and direct mechanisms, involving binding to AMPK subunits to promote activation. By switching off anabolic processes and stimulating catabolic pathways, AMPK plays a critical role in managing metabolic disorders, cancer, and cardiovascular disease. This blog explores the structural basis of AMPK regulation, the mechanisms of activator classes, and future directions for therapeutic development, emphasizing the pathway’s central role as a master metabolic switch.
Introduction — AMPK as the Master Metabolic Switch
Cells require a constant supply of energy to sustain life, and the coordination of energy production with energy demand is a fundamental aspect of cellular physiology. At the center of this balance is AMP-activated protein kinase (AMPK), often described as the master regulator of cellular energy homeostasis. Acting as a cellular “thermostat,” AMPK senses changes in energy availability and rapidly adjusts metabolic pathways to restore equilibrium. When cellular energy levels drop, reflected by increased ratios of AMP or ADP to ATP, AMPK is activated and initiates a cascade of metabolic adjustments that favor energy production while conserving energy expenditure.
AMPK is a heterotrimeric enzyme composed of three subunits: the catalytic α-subunit, a scaffolding β-subunit, and a regulatory γ-subunit. Its activation requires phosphorylation at threonine-172 of the α-subunit, typically by upstream kinases such as liver kinase B1 (LKB1) or calcium/calmodulin-dependent kinase kinase β (CaMKKβ). Additionally, AMP and ADP binding to the γ-subunit protects AMPK from dephosphorylation and promotes further activation. This dual regulatory mechanism enables AMPK to serve as a sensitive detector of cellular energy stress.
Once activated, AMPK acts as a metabolic switch, turning off anabolic processes that consume ATP, such as lipid synthesis, protein synthesis, and gluconeogenesis, while turning on catabolic pathways that generate ATP, including fatty acid oxidation, glycolysis, and autophagy. This adaptive response ensures that energy resources are mobilized efficiently during times of metabolic stress. Beyond short-term energy balance, AMPK also exerts long-term effects by regulating gene expression and mitochondrial biogenesis, further highlighting its importance in maintaining metabolic health.
The significance of AMPK extends beyond basic physiology. Dysregulation of AMPK signaling is implicated in the pathogenesis of several diseases, including type 2 diabetes, obesity, cardiovascular disease, and cancer. Consequently, AMPK has become a major therapeutic target, with both natural compounds and synthetic small molecules being investigated as activators. Understanding AMPK’s central role as a master metabolic switch not only provides insights into fundamental biology but also offers a framework for designing interventions to combat metabolic and proliferative disorders.
How AMPK Works: Structure and Function
AMP-activated protein kinase (AMPK) is a heterotrimeric complex that integrates signals of cellular energy status and coordinates adaptive metabolic responses. Its structure consists of three subunits—α, β, and γ—each performing distinct but complementary roles. The α-subunit serves as the catalytic core, containing the kinase domain responsible for phosphorylating downstream targets. A critical site within this domain is threonine-172 (Thr172), whose phosphorylation is essential for AMPK activation. The β-subunit functions as a scaffold, facilitating assembly of the α and γ subunits while also containing a carbohydrate-binding module that may anchor the complex to glycogen particles. The γ-subunit provides regulatory control through its four cystathionine β-synthase (CBS) domains, which bind adenine nucleotides (AMP, ADP, and ATP).
Activation of AMPK is tightly regulated through both allosteric mechanisms and post-translational modification. When cellular ATP levels fall, AMP and ADP bind to the γ-subunit at distinct CBS domains. This binding event not only allosterically activates the kinase but also shields Thr172 from dephosphorylation, prolonging AMPK activity. Conversely, high ATP levels compete with AMP and ADP for binding, keeping AMPK in an inactive state. Phosphorylation of Thr172 by upstream kinases provides the decisive activation step. The tumor suppressor liver kinase B1 (LKB1) is the primary upstream kinase under conditions of metabolic stress, while calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ) can activate AMPK in response to calcium signaling, linking AMPK to pathways beyond energy depletion.
Once activated, AMPK orchestrates a switch in cellular metabolism by inhibiting ATP-consuming anabolic processes while promoting ATP-generating catabolic pathways. For example, AMPK phosphorylates and inactivates acetyl-CoA carboxylase (ACC), thereby reducing fatty acid synthesis and enhancing fatty acid oxidation. It also inhibits the mechanistic target of rapamycin complex 1 (mTORC1), suppressing protein synthesis. Simultaneously, AMPK stimulates glucose uptake through translocation of glucose transporter 4 (GLUT4) and enhances mitochondrial biogenesis by activating peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α).
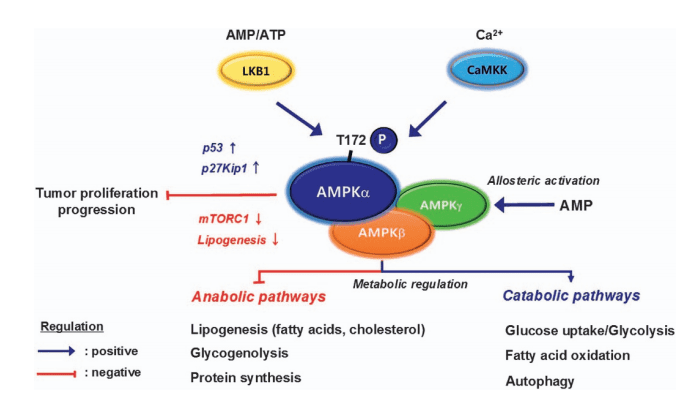
Figure 1 A summary of the physiological roles of AMP-activated protein kinase (AMPK).
In addition to acute metabolic adjustments, AMPK exerts longer-term control by influencing transcriptional programs and epigenetic regulation. This dual role underscores its central importance in cellular physiology. By coupling energy sensing with metabolic reprogramming, AMPK ensures that cells maintain homeostasis under fluctuating nutrient and energy conditions.
Indirect AMPK Activators — Harnessing Cellular Stress
Not all AMP-activated protein kinase (AMPK) activators act by binding directly to the complex. Many compounds instead activate AMPK indirectly, by altering cellular energy balance or related signaling cascades. These indirect activators raise the AMP:ATP or ADP:ATP ratio, or increase intracellular calcium, thereby engaging AMPK’s natural sensing mechanisms. Their pharmacological diversity makes them particularly interesting in both research and therapeutic contexts.
Metformin and thiazolidinediones (TZDs) are two of the most notable clinically used AMPK activators. Metformin, a first-line therapy for type 2 diabetes, inhibits mitochondrial respiratory complex I, leading to reduced ATP synthesis and a subsequent rise in AMP. This increase activates AMPK, which then enhances glucose uptake and reduces hepatic gluconeogenesis. Similarly, TZDs, used in insulin resistance, indirectly activate AMPK through mitochondrial effects and by elevating adiponectin signaling, which stimulates AMPK via upstream kinases.
Natural products and polyphenols also form a significant group of indirect AMPK activators. Compounds such as resveratrol, epigallocatechin gallate (EGCG), quercetin, and berberine are widely studied for their effects on energy metabolism. Like metformin, many of these agents inhibit components of the mitochondrial electron transport chain, thus promoting AMPK activation. In addition, curcumin and ginsenosides (e.g., Rb1) can modulate AMPK activity via both energy stress and calcium-mediated pathways.
Another mechanism involves oxidative stress and reactive oxygen species (ROS). Compounds such as cryptotanshinone stimulate AMPK in part by inducing mild oxidative stress, which secondarily disrupts mitochondrial function. Interestingly, α-lipoic acid is able to activate AMPK through CaMKKβ-dependent signaling, even in the absence of significant changes in adenine nucleotide ratios, highlighting the diversity of indirect pathways.
The broad range of indirect activators underscores AMPK’s centrality as a metabolic hub responsive to multiple stress inputs. While indirect activators lack the precise subunit selectivity of direct small molecules, their systemic effects often provide therapeutic advantages. For example, polyphenols combine AMPK activation with antioxidant and anti-inflammatory actions, making them attractive leads in metabolic disease and cancer research.
By harnessing mitochondrial stress, calcium signaling, and redox balance, indirect activators illustrate how diverse metabolic pathways converge on AMPK. This class of compounds not only illuminates fundamental biology but also continues to inspire drug discovery for disorders of metabolism and energy dysregulation.
Direct AMPK Activators — Precision Molecular Tools
While many compounds activate AMP-activated protein kinase (AMPK) indirectly through mitochondrial or calcium signaling, a distinct class of direct activators interacts with AMPK itself, binding to specific subunits or domains. These molecules offer more precision, as they can modulate AMPK without broadly perturbing cellular energy status. For researchers and drug developers, direct activators provide a powerful tool to probe AMPK biology and develop therapeutic strategies with fewer off-target effects.
One of the earliest direct activators identified was AICAR (5-aminoimidazole-4-carboxamide ribonucleotide). Once taken up by cells, AICAR is phosphorylated to ZMP, an AMP analog that binds to the γ-subunit at the nucleotide-binding sites. This binding mimics natural allosteric activation by AMP, promoting phosphorylation of Thr172 and inhibiting dephosphorylation. Although AICAR has been widely used in experimental studies, its poor pharmacokinetic properties have limited therapeutic applications.
A significant advance came with compounds that target the β-subunit carbohydrate-binding module (CBM). A-769662 and structurally related molecules, such as compound 991, bind at the interface between the β-subunit CBM and the α-subunit kinase domain. This interaction not only allosterically activates AMPK but also protects Thr172 from dephosphorylation, prolonging activity. Interestingly, these agents show isoform selectivity, being most potent against AMPK complexes containing the β1 isoform. This feature provides opportunities for tissue-specific modulation, as β1 expression is enriched in certain metabolic tissues.
Another notable example is salicylate, the active metabolite of aspirin, which has been shown to directly activate AMPK through the same CBM binding site as A-769662. Its dual role as an anti-inflammatory and AMPK activator links classic pharmacology with modern metabolic regulation.
More recent discoveries include compound-13 (C-13), a prodrug of C-2 that selectively activates AMPK complexes containing the α1 subunit, and MT 63-78, a thienopyridone derivative that stimulates AMPK without altering adenine nucleotide ratios. These examples demonstrate the chemical diversity of direct activators and their ability to target different subunit combinations for tailored effects.
Direct activators represent a promising frontier in AMPK research and drug development. By bypassing the need to induce cellular stress, they offer greater specificity, reduced side effects, and potential for combination therapy with indirect activators. As structural biology advances, the rational design of direct AMPK activators is likely to accelerate, providing refined tools to harness this central metabolic switch.
Therapeutic Implications and Future Directions
AMP-activated protein kinase (AMPK) sits at the crossroads of metabolism, making it an attractive therapeutic target across a wide range of diseases. By sensing cellular energy deficits and promoting adaptive responses, AMPK activation offers benefits in conditions where energy homeostasis is disrupted, such as type 2 diabetes, obesity, cardiovascular disease, and cancer. The diverse portfolio of indirect and direct activators highlights both the therapeutic promise and the complexity of translating AMPK biology into clinical strategies.
In metabolic disease, AMPK activation has well-established benefits. Drugs such as metformin already exploit AMPK pathways to improve glycemic control and insulin sensitivity. Beyond glucose metabolism, AMPK reduces hepatic lipid synthesis, enhances fatty acid oxidation, and promotes mitochondrial health, making it a potential intervention for non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome. Lifestyle interventions such as exercise also rely, at least in part, on AMPK signaling, underscoring its role as a molecular mediator of systemic metabolic fitness.
In oncology, AMPK’s role is more nuanced. By inhibiting anabolic processes, AMPK can suppress tumor cell growth and proliferation. Compounds such as MT 63-78 demonstrate that direct AMPK activation can reduce lipogenesis and slow tumor progression, particularly in cancers reliant on lipid biosynthesis. However, cancer cells may also adapt to AMPK activation by enhancing survival under metabolic stress, suggesting that therapeutic strategies must be carefully tailored to tumor context.
Cardiovascular research further underscores the potential of AMPK activators. By improving endothelial function, reducing oxidative stress, and enhancing myocardial energy balance, AMPK-targeting agents may mitigate the progression of atherosclerosis and protect against ischemic injury. Polyphenols such as resveratrol and quercetin highlight how natural AMPK activators could complement pharmacological strategies in cardiovascular prevention.
Looking ahead, combination therapies may prove especially effective. For example, pairing indirect activators such as metformin with direct activators like salicylate or A-769662 could yield synergistic benefits by engaging multiple regulatory mechanisms. Advances in structural biology and high-throughput screening are also enabling the development of isoform-selective compounds, which may allow for tissue-specific AMPK modulation and reduced side effects.
In summary, AMPK activators hold promise as next-generation therapeutics that extend beyond traditional metabolic disease into oncology and cardiovascular medicine. With continued progress in drug design and clinical translation, targeting AMPK may emerge as a unifying approach to treating diseases rooted in disordered energy metabolism.
References
Hardie, D. G., Ross, F. A., & Hawley, S. A. (2012). AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology, 13(4), 251–262.
https://doi.org/10.1038/nrm3311
Hardie, D. G., Schaffer, B. E., & Brunet, A. (2016). AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends in Cell Biology, 26(3), 190–201.
https://doi.org/10.1016/j.tcb.2015.10.013
Herzig, S., & Shaw, R. J. (2018). AMPK: Guardian of metabolism and mitochondrial homeostasis. Nature Reviews Molecular Cell Biology, 19(2), 121–135.
https://doi.org/10.1038/nrm.2017.95
Steinberg, G. R., & Carling, D. (2019). AMP-activated protein kinase: The current landscape for drug development. Nature Reviews Drug Discovery, 18(7), 527–551.
https://doi.org/10.1038/s41573-019-0019-2
Carling, D., Thornton, C., Woods, A., & Sanders, M. J. (2012). AMP-activated protein kinase: new regulation, new roles? Biochemical Journal, 445(1), 11–27.
https://doi.org/10.1042/BJ20120546
Hardie, D. G. (2014). AMPK—sensing energy while talking to other signaling pathways. Cell Metabolism, 20(6), 939–952.
https://doi.org/10.1016/j.cmet.2014.09.013
Ross, F. A., MacKintosh, C., & Hardie, D. G. (2016). AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS Journal, 283(16), 2987–3001.
https://doi.org/10.1111/febs.13698
Xiao, B., Sanders, M. J., Carmena, D., Bright, N. J., Haire, L. F., Underwood, E., … & Gamblin, S. J. (2011). Structural basis of AMPK regulation by small molecule activators. Nature, 472(7342), 230–233.
https://doi.org/10.1038/nature09932
Hardie, D. G. (2015). AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Current Opinion in Cell Biology, 33, 1–7.
https://doi.org/10.1016/j.ceb.2014.09.004
Hawley, S. A., Gadalla, A. E., Olsen, G. S., & Hardie, D. G. (2002). The antidiabetic drug metformin activates the AMP-activated protein kinase cascade in hepatocytes. Diabetes, 51(8), 2420–2425. https://doi.org/10.2337/diabetes.51.8.2420
Kim, J., Yang, G., Kim, Y., Kim, J., & Ha, J. (2016). AMPK activators: mechanisms of action and physiological activities. Experimental & Molecular Medicine, 48(4), e224.
https://doi.org/10.1038/emm.2016.16
Zang, M., Xu, S., Maitland-Toolan, K. A., Zuccollo, A., Hou, X., Jiang, B., … & Ruderman, N. B. (2006). Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor–deficient mice. Diabetes, 55(8), 2180–2191.
https://doi.org/10.2337/db05-1188
Cool, B., Zinker, B., Chiou, W., Kifle, L., Cao, N., Perham, M., … & Ewing, W. (2006). Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metabolism, 3(6), 403–416.
https://doi.org/10.1016/j.cmet.2006.05.005
Zadra, G., Photopoulos, C., Tyekucheva, S., Heidari, P., Weng, Q. P., Fedele, G., … & Loda, M. (2014). A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Molecular Medicine, 6(4), 519–538.