AMPK Activators: Unlocking New Pathways in Metabolic and Cancer Therapy
Abstract
AMP-activated protein kinase (AMPK) is a pivotal energy sensor that regulates cellular metabolism, stress response, and homeostasis. With mounting evidence supporting its role in the pathogenesis of type 2 diabetes, obesity, and cancer, AMPK has become an attractive target for therapeutic intervention. This blog explores the molecular mechanisms of AMPK activation, distinguishing between indirect activators—such as metformin and polyphenols that alter intracellular AMP:ATP ratios—and direct activators like A-769662 and salicylate, which bind specific AMPK subunits to induce activity. The post highlights AMPK’s dual role in metabolic regulation and tumor suppression, discusses isoform-specific modulation strategies, and examines emerging combinatorial therapies. As AMPK-targeting compounds progress from preclinical models to clinical potential, their integration into metabolic and oncologic treatment strategies may offer novel and more effective therapies.
AMPK at the Center of Cellular Energy Control
In the ever-evolving landscape of metabolic research, AMP-activated protein kinase (AMPK) has emerged as a central player in cellular energy regulation. Often described as a cellular “fuel gauge,” AMPK senses fluctuations in intracellular energy levels and acts swiftly to restore balance. When energy is depleted—such as during exercise, nutrient scarcity, or metabolic stress—AMPK activates pathways that generate ATP while simultaneously shutting down energy-consuming processes like lipid synthesis and protein translation.
AMPK is a heterotrimeric complex composed of catalytic (α), scaffolding (β), and regulatory (γ) subunits, each existing in multiple isoforms. This structural complexity allows for tissue-specific and stimulus-specific responses, making AMPK a versatile target in both physiology and pharmacology. The kinase is activated by two major mechanisms: allosteric binding of AMP or ADP to the γ subunit, and phosphorylation of a key threonine residue (Thr-172) on the α subunit by upstream kinases such as LKB1 or CaMKKβ. These dual mechanisms ensure that AMPK can fine-tune energy homeostasis under diverse cellular conditions.
The implications of AMPK activation extend beyond energy metabolism. Over the past two decades, AMPK has been implicated in several pathophysiological processes, including type 2 diabetes, obesity, cardiovascular disease, and even cancer. Its ability to inhibit anabolic pathways and promote autophagy positions it as a promising therapeutic target. Importantly, both natural and synthetic compounds capable of modulating AMPK activity are under intense investigation, reflecting growing interest in AMPK-based drug development.
As the understanding of AMPK’s regulatory networks deepens, so does the potential for novel therapies that harness its energy-sensing capabilities. Whether the goal is to restore insulin sensitivity or suppress tumor growth, targeting AMPK offers a unified approach to managing diseases rooted in metabolic dysfunction.
Indirect AMPK Activators – Targeting the AMP/ATP Ratio
Indirect AMPK activators represent a broad class of compounds that stimulate AMPK activity by altering the cellular energy status—typically by increasing the AMP:ATP or ADP:ATP ratio. Unlike direct activators that bind to the AMPK complex itself, these compounds induce AMPK activation by mimicking metabolic stress. As a result, they initiate phosphorylation of AMPK via upstream kinases like LKB1 and prevent dephosphorylation, making them potent tools in targeting energy metabolism.
One of the most studied indirect activators is metformin, a biguanide used as a first-line treatment for type 2 diabetes. Metformin inhibits mitochondrial respiratory complex I, thereby decreasing ATP levels and increasing intracellular AMP. This shift activates AMPK, which then promotes fatty acid oxidation, reduces hepatic glucose production, and improves insulin sensitivity.
Another pharmaceutical group with indirect AMPK activity is the thiazolidinediones (TZDs), such as pioglitazone and rosiglitazone. While they are known for activating PPARγ, TZDs also stimulate AMPK in tissues like liver, muscle, and adipose tissue. Part of their effect is mediated through increased AMP levels and the adipokine adiponectin, which itself is a known AMPK activator.
In addition to synthetic drugs, several natural polyphenols—including resveratrol, quercetin, berberine, and EGCG—indirectly activate AMPK. These compounds often inhibit mitochondrial ATP production, thereby elevating AMP and mimicking low-energy conditions. For instance, resveratrol inhibits ATP synthase, while berberine targets respiratory chain complex I.
Other bioactive compounds like α-lipoic acid (ALA) and ginsenosides from ginseng also stimulate AMPK, often through calcium-mediated signaling or mitochondrial disruption. The variety of mechanisms among indirect activators highlights the complexity and flexibility of AMPK as a metabolic sensor.
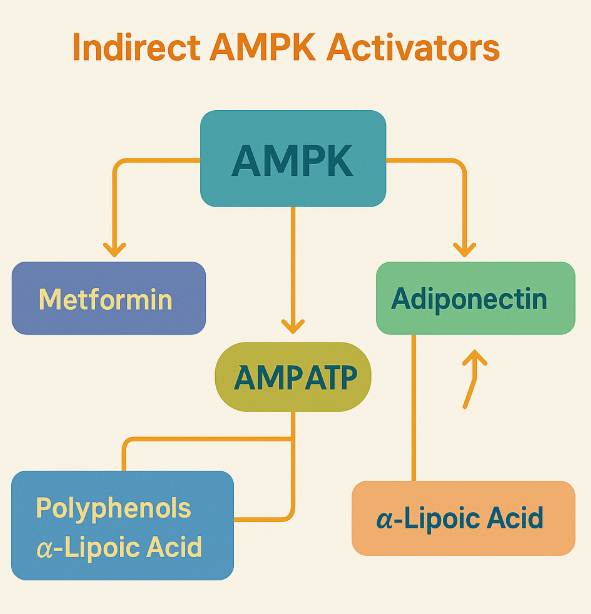
Fig.1 Indirect AMPK Activators
These compounds collectively support AMPK’s role as a viable therapeutic target in managing metabolic diseases. Their indirect mechanisms may also offer synergy when combined with direct AMPK activators, broadening therapeutic strategies for disorders like obesity, insulin resistance, and cardiovascular disease.
Direct AMPK Activators – Precision Activation at the Molecular Level
Unlike indirect activators that trigger AMPK through shifts in cellular energy levels, direct AMPK activators bind to specific sites on the AMPK complex itself. These molecules often work without changing intracellular AMP, ADP, or ATP concentrations. Their ability to selectively activate AMPK through defined subunit interactions opens the door to isoform-specific modulation, a key advantage for precision medicine and targeted metabolic therapies.
One of the earliest known direct activators is AICAR (5-aminoimidazole-4-carboxamide ribonucleoside), an adenosine analog. Once inside the cell, AICAR is converted to ZMP, a compound that mimics AMP and binds to the γ subunit of AMPK. Although widely used in research, AICAR’s broad activity on AMP-sensitive enzymes limits its therapeutic use.
A significant breakthrough came with the discovery of A-769662, a thienopyridone derivative that allosterically activates AMPK and prevents dephosphorylation at Thr-172 on the α subunit. Notably, A-769662 specifically targets complexes containing the β1 subunit, particularly at Ser108, and does not require upstream kinase activity for AMPK activation. This makes it a more selective and potent tool compared to nucleotide mimetics.
Another potent molecule is Compound 991, which shares a similar binding site and mechanism with A-769662 but exhibits greater potency. Both compounds bind at the interface between the α subunit’s kinase domain and the carbohydrate-binding module (CBM) of the β1 subunit, underscoring the importance of AMPK’s modular structure in drug design.
Salicylate, the active metabolite of aspirin, has also been shown to directly activate AMPK at the same β1-dependent site as A-769662. Interestingly, salicylate antagonizes A-769662’s effects in competitive binding studies, suggesting overlapping interaction sites.
These direct activators offer key advantages in specificity and efficacy, particularly in tissues or tumors where upstream kinases like LKB1 are dysfunctional. Their isoform selectivity also allows for tailored intervention strategies in diseases ranging from diabetes to cancer.
AMPK in Disease – From Diabetes to Cancer
AMPK’s role extends far beyond cellular metabolism—it is a key integrator of energy status in whole-body physiology, with profound implications for treating chronic diseases like type 2 diabetes, obesity, and cancer. As a central metabolic regulator, AMPK activation promotes beneficial adaptations such as enhanced glucose uptake, fatty acid oxidation, and mitochondrial biogenesis, while simultaneously inhibiting energy-intensive processes like lipid and protein synthesis.
In metabolic disorders such as type 2 diabetes, AMPK activation helps improve insulin sensitivity and reduce hepatic glucose production. Indirect activators like metformin and thiazolidinediones (TZDs) owe part of their therapeutic action to AMPK modulation. AMPK suppresses key gluconeogenic genes and promotes catabolic pathways, making it a valuable target for correcting energy imbalances in diabetic patients.
Beyond metabolism, AMPK has emerged as a critical player in tumor suppression. One of the earliest links between AMPK and cancer was the discovery that LKB1, a tumor suppressor frequently mutated in Peutz-Jeghers syndrome and many sporadic cancers, is a major upstream kinase that activates AMPK. Through this pathway, AMPK can inhibit the mammalian target of rapamycin (mTOR), a driver of protein synthesis and cell proliferation, effectively serving as a brake on uncontrolled cell growth.
AMPK activation can also induce cell cycle arrest via p53 and p21, promote autophagy, and reduce lipogenesis, which is often elevated in rapidly growing tumors. However, this role is context-dependent; once a tumor is established, AMPK may help cancer cells adapt to metabolic stress, making its therapeutic targeting complex.
Still, evidence from animal models and cell lines suggests that direct AMPK activators—especially those that bypass upstream kinase defects—may offer therapeutic value in LKB1-deficient tumors and in combination with lipogenesis-targeting therapies. This dual utility in both metabolic and cancer biology highlights AMPK as a versatile and compelling drug target.
Future Directions – Combinatorial Therapies and Drug Development
As research into AMP-activated protein kinase (AMPK) deepens, so does the potential to exploit it as a multifaceted therapeutic target. While current treatments using indirect activators like metformin have validated the therapeutic benefit of AMPK modulation, the future lies in refining these approaches through combination therapies and isoform-selective drug development.
One promising avenue is the use of combinatorial therapies that pair AMPK activators with other metabolic or oncologic agents. For example, studies have demonstrated a synergistic effect between metformin and salicylate, where the combination significantly impairs the survival of prostate and lung cancer cells by inhibiting de novo lipogenesis. This finding highlights how dual activation of AMPK through different mechanisms—metformin indirectly via AMP accumulation and salicylate directly via β1 subunit binding—can enhance therapeutic efficacy.
Furthermore, direct AMPK activators offer a solution to a significant clinical challenge: tumors with LKB1 mutations, which render them unresponsive to AMP-dependent activation pathways. Compounds such as A-769662, Compound 991, and MT 63–78 have shown effectiveness in bypassing the need for upstream kinase signaling, providing a route to target LKB1-deficient cancers that account for a significant portion of non-small cell lung cancers and other malignancies.
The development of isoform-specific AMPK activators is also gaining traction. Compounds targeting specific combinations like α1β1γ1 or α2β2γ3 are under investigation to achieve tissue-selective responses—an important step toward reducing side effects and enhancing clinical outcomes in metabolic or cancer therapy.
As we look ahead, the integration of AMPK modulation with personalized medicine strategies and lipid metabolism inhibitors may redefine how we approach treatment for chronic diseases rooted in metabolic dysfunction. The versatility and druggability of AMPK make it a compelling focus for next-generation therapeutic design.
References:
Hardie, D. G., Ross, F. A., & Hawley, S. A. (2012). AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology, 13(4), 251–262.
https://doi.org/10.1038/nrm3311
Mihaylova, M. M., & Shaw, R. J. (2011). The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biology, 13(9), 1016–1023.
https://doi.org/10.1038/ncb2329
Kim, J., Yang, G., Kim, Y., Kim, J., & Ha, J. (2016). AMPK activators: Mechanisms of action and physiological activities. Experimental & Molecular Medicine, 48(4), e224.
https://doi.org/10.1038/emm.2016.16
Oakhill, J. S., Steel, R., Chen, Z. P., Scott, J. W., Ling, N., Tam, S., & Kemp, B. E. (2011). AMPK is a direct adenylate charge-regulated protein kinase. Science, 332(6036), 1433–1435.
https://doi.org/10.1126/science.1200094
Kim, J., Yang, G., Kim, Y., Kim, J., & Ha, J. (2016). AMPK activators: Mechanisms of action and physiological activities. Experimental & Molecular Medicine, 48(4), e224.
https://doi.org/10.1038/emm.2016.16
Zhou, G., Myers, R., Li, Y., Chen, Y., Shen, X., Fenyk-Melody, J., … & Moller, D. E. (2001). Role of AMP-activated protein kinase in mechanism of metformin action. Journal of Clinical Investigation, 108(8), 1167–1174.
https://doi.org/10.1172/JCI13505
Turner, N., Li, J. Y., Gosby, A., To, S. W., Cheng, Z., Miyoshi, H., … & Cooney, G. J. (2008). Berberine and its more biologically available derivative inhibit mitochondrial respiratory complex I: a mechanism for AMPK activation. Diabetes, 57(5), 1414–1420.
https://doi.org/10.2337/db07-1552
Lee, W. J., Song, K. H., Koh, E. H., Won, J. C., Kim, H. S., Park, H. S., … & Kim, M. S. (2005). Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochemical and Biophysical Research Communications, 332(3), 885–891.
https://doi.org/10.1016/j.bbrc.2005.05.038
Kim, J., Yang, G., Kim, Y., Kim, J., & Ha, J. (2016). AMPK activators: Mechanisms of action and physiological activities. Experimental & Molecular Medicine, 48(4), e224.
https://doi.org/10.1038/emm.2016.16
Cool, B., Zinker, B., Chiou, W., Kifle, L., Cao, N., Perham, M., … & Frevert, E. (2006). Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metabolism, 3(6), 403–416.
https://doi.org/10.1016/j.cmet.2006.05.005
Xiao, B., Sanders, M. J., Carmena, D., Bright, N. J., Haire, L. F., Underwood, E., … & Carling, D. (2013). Structural basis of AMPK regulation by small molecule activators. Nature Communications, 4, 3017.
https://doi.org/10.1038/ncomms4017
Hawley, S. A., Fullerton, M. D., Ross, F. A., Schertzer, J. D., Chevtzoff, C., Walker, K. J., … & Hardie, D. G. (2012). The ancient drug salicylate directly activates AMP-activated protein kinase. Science, 336(6083), 918–922.
https://doi.org/10.1126/science.1215327
Shackelford, D. B., & Shaw, R. J. (2009). The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nature Reviews Cancer, 9(8), 563–575.
https://doi.org/10.1038/nrc2676
O’Brien, A. J., Villani, L. A., Broadfield, L. A., Houde, V. P., Galic, S., Blandino, G., … & St-Pierre, J. (2015). Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochemical Journal, 469(1), 177–187.