AMG 410 and the Next Frontier in KRAS-Driven Cancer Treatment
Abstract
KRAS mutations are among the most prevalent oncogenic drivers in solid tumors, yet they have long been considered undruggable. AMG 410 represents a new generation of KRAS inhibitors designed to overcome these challenges. Unlike covalent, mutation-specific agents, AMG 410 is a non-covalent, state-independent inhibitor capable of targeting multiple KRAS mutations, including G12D, G12V, and G13D. It binds both GTP- and GDP-bound KRAS, offering broad-spectrum suppression of oncogenic signaling. With over 100-fold selectivity for KRAS over HRAS and NRAS, AMG 410 avoids unwanted antiproliferative effects in non-KRAS-driven cells and shows potent efficacy in preclinical models of colorectal, pancreatic, and lung cancers. Furthermore, AMG 410 demonstrates synergy with targeted therapies and immunotherapies, while maintaining a favorable safety profile. As it moves toward clinical trials, AMG 410 holds promise as a first-in-class therapeutic for KRAS-driven malignancies, potentially transforming treatment strategies in precision oncology.
Introduction
KRAS, one of the most frequently mutated oncogenes in human cancers, has long represented a formidable challenge in precision oncology. Mutations in KRAS drive tumor growth in a variety of solid tumors, including non-small cell lung cancer (NSCLC), colorectal cancer, and pancreatic ductal adenocarcinoma (PDAC), contributing to aggressive disease progression and poor clinical outcomes. Despite the well-established role of KRAS in oncogenesis, efforts to therapeutically target it were historically considered futile, earning KRAS the reputation of being “undruggable” due to its high affinity for GTP/GDP and lack of suitable binding pockets.

Recent breakthroughs, however, have led to the development of selective KRAS inhibitors such as sotorasib and adagrasib, which target the KRAS G12C mutation through covalent binding. While these agents have shown promising clinical activity, their mutation-specific nature and limitation to GDP-bound KRAS restrict their applicability. The diversity of KRAS mutations—such as G12D, G12V, and G13D—presents a significant unmet need for broader-spectrum inhibitors that can effectively suppress multiple KRAS variants and overcome state-dependency.
Enter AMG 410, a novel non-covalent KRAS inhibitor that represents a significant leap forward in targeted cancer therapy. Designed to engage both the active (GTP-bound) and inactive (GDP-bound) forms of KRAS, AMG 410 achieves state-independent inhibition across a broad range of KRAS mutations. This capability positions AMG 410 as a promising candidate for treating a wider population of patients with KRAS-mutant tumors, potentially transforming therapeutic strategies in oncology.
The Challenge of KRAS-Driven Cancers
KRAS mutations are among the most prevalent oncogenic drivers across various solid tumors, yet they remain notoriously difficult to treat. Approximately 25% of all human cancers harbor mutations in RAS genes, with KRAS accounting for over 85% of these cases. These mutations are particularly common in pancreatic cancer (90%), colorectal cancer (40%), and non-small cell lung cancer (30%), underscoring their central role in tumor initiation and progression .
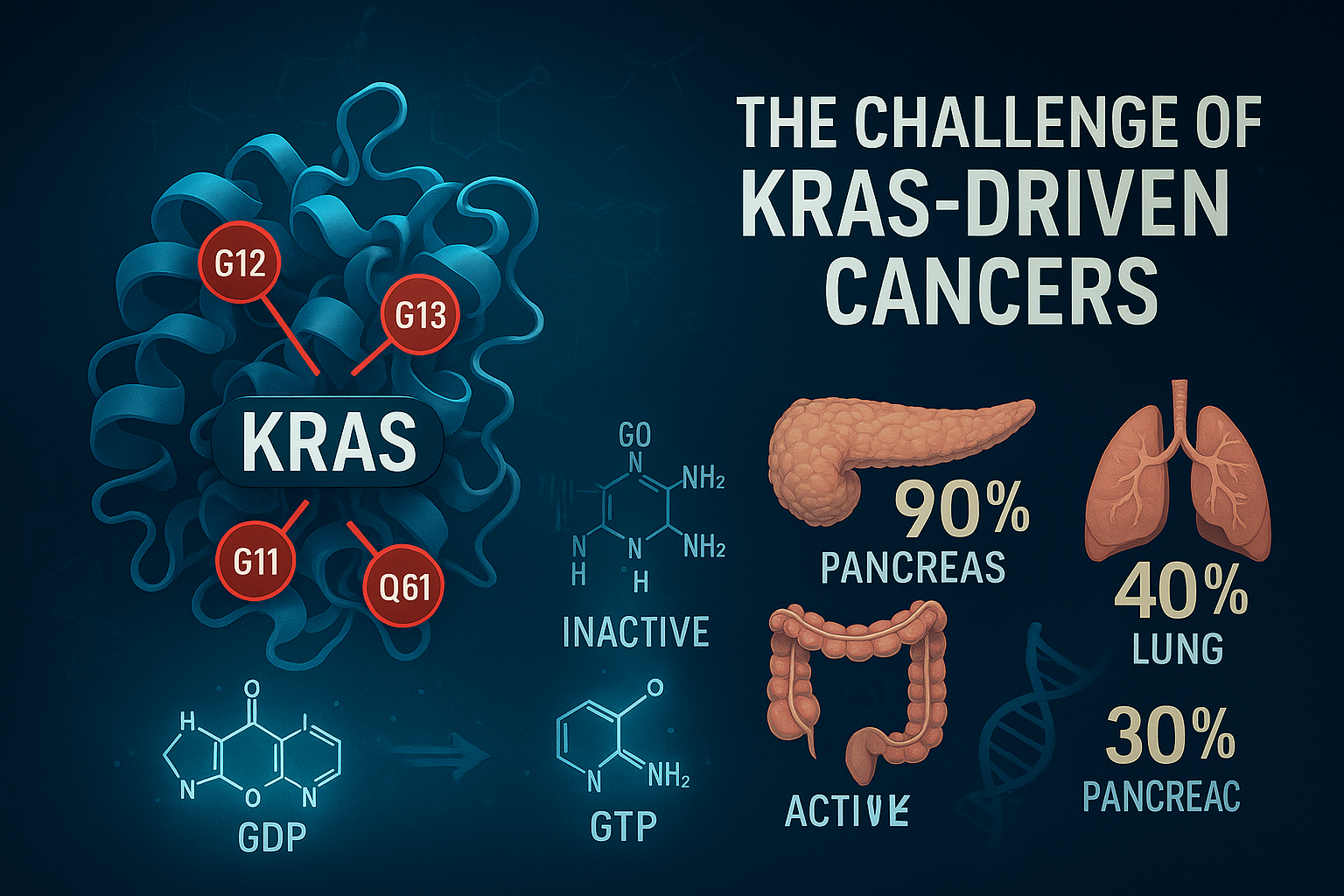
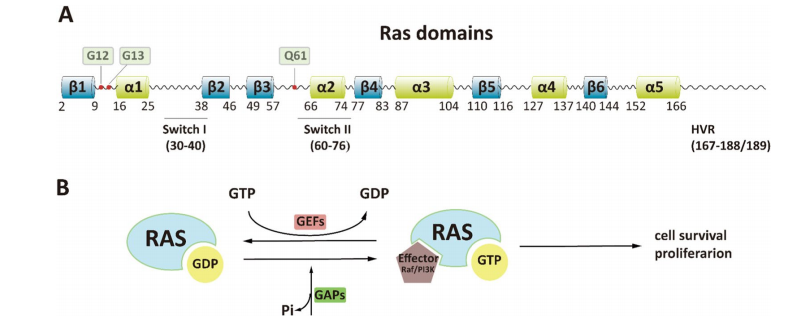
KRAS proteins function as molecular switches, cycling between an active GTP-bound state and an inactive GDP-bound state to regulate key cellular pathways such as MAPK/ERK and PI3K/AKT. Oncogenic mutations—most commonly at codons G12, G13, and Q61—lock KRAS in its active state, resulting in uncontrolled cell proliferation and resistance to apoptosis.
Historically, KRAS was considered “undruggable” due to its smooth, pocketless surface and the picomolar affinity of GTP/GDP binding. Early attempts to inhibit KRAS directly were unsuccessful, pushing researchers to explore indirect approaches such as targeting downstream effectors or synthetic lethality networks. While selective inhibitors like sotorasib (targeting KRAS G12C) marked a milestone, they are only effective in a small fraction of patients and depend on the KRAS GDP-bound inactive form.
The field now demands next-generation inhibitors that can tackle multiple KRAS mutations across both active and inactive states—an unmet need that AMG 410 is uniquely designed to fulfill.
AMG 410 – Mechanism of Action
AMG 410 is a novel non-covalent KRAS inhibitor designed to overcome the limitations of mutation- and state-specific agents. Its standout feature is its ability to bind both the GTP-bound (active) and GDP-bound (inactive) forms of KRAS, allowing for state-independent and sustained inhibition of oncogenic signaling. This dual-targeting mechanism enables AMG 410 to suppress a broader array of KRAS mutations, including G12D, G12V, and G13D, which are not addressed by current approved therapies.
Unlike covalent inhibitors such as sotorasib and adagrasib that lock onto KRAS G12C via the cysteine residue, AMG 410 uses non-covalent interactions to engage the switch II pocket. This approach allows for reversible binding, which can reduce potential resistance mechanisms associated with irreversible inhibition.
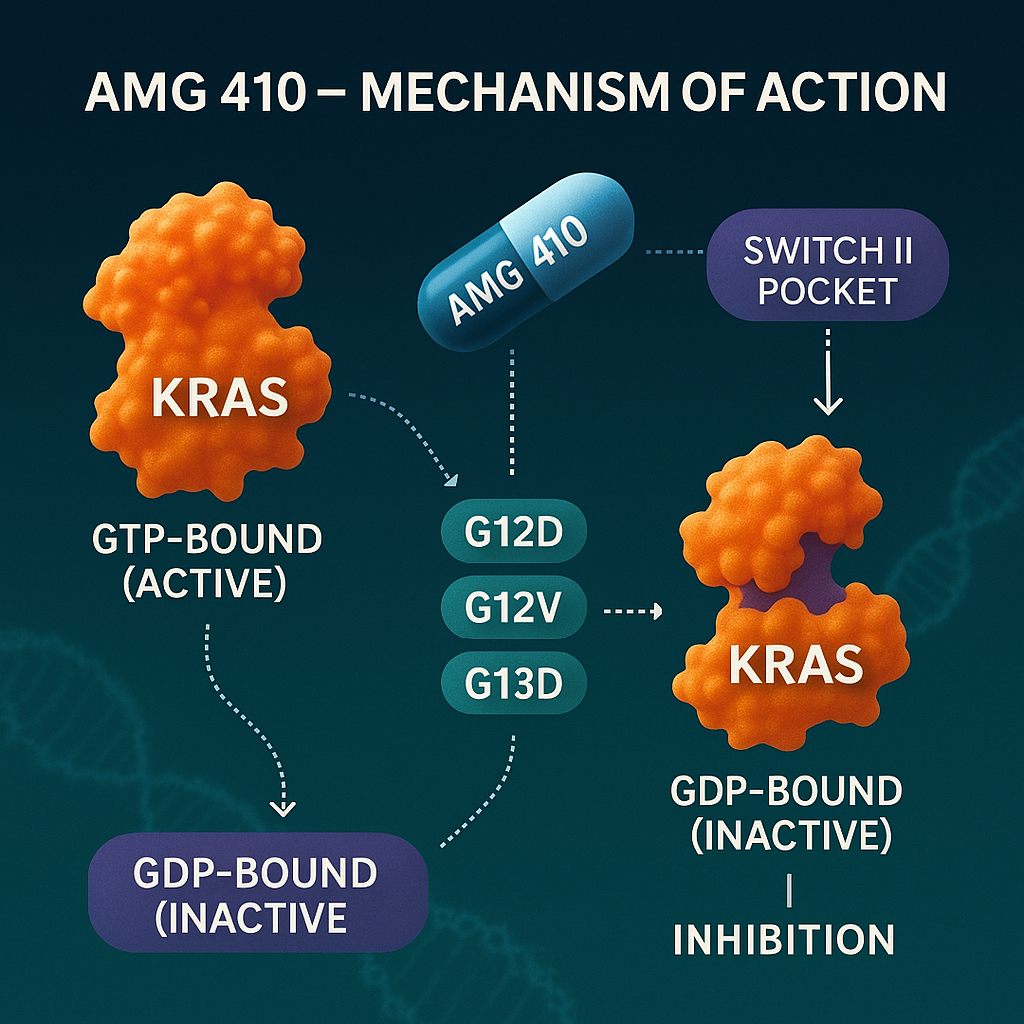
Another key distinction lies in AMG 410’s high selectivity for KRAS over other RAS isoforms. It shows >100-fold selectivity against HRAS and NRAS, minimizing off-target toxicity in non-KRAS-driven cells—a critical consideration for therapeutic safety. This precision allows AMG 410 to inhibit oncogenic KRAS signaling while sparing normal cellular processes.
By inhibiting downstream ERK phosphorylation, AMG 410 disrupts the KRAS-RAF-MEK-ERK cascade that drives tumor growth. In preclinical models of KRAS-mutant lung, colorectal, and pancreatic cancers, AMG 410 demonstrated robust antitumor activity, including stable tumor shrinkage and synergistic effects in combination therapies.
Selectivity and Safety Advantages
A major challenge in the development of RAS inhibitors has been achieving specificity—particularly distinguishing between the three highly homologous isoforms: KRAS, NRAS, and HRAS. AMG 410 sets itself apart with its exceptionally high selectivity for KRAS, exhibiting >100-fold lower activity against NRAS and HRAS. This design helps mitigate the risk of disrupting normal RAS-dependent cellular functions in non-tumor tissues.
This selectivity is significant because pan-RAS inhibition can lead to undesirable cytotoxicity in healthy cells. The RAS family is essential for numerous physiological processes, and broad inhibition can compromise hematopoietic, cardiovascular, and immune function. AMG 410’s focused mechanism helps avoid these effects, especially in non-KRAS-driven cells, where the median IC₅₀ exceeds 5 µM, indicating minimal antiproliferative activity.
Furthermore, the non-covalent and reversible binding profile of AMG 410 is expected to provide better pharmacokinetic flexibility and reduced resistance liability. Covalent KRAS G12C inhibitors, while effective in some contexts, can trigger rapid resistance through secondary mutations or pathway reactivation. AMG 410’s mutation-agnostic and state-independent inhibition could reduce these escape mechanisms.
From a safety standpoint, AMG 410’s preclinical evaluations demonstrate a favorable therapeutic index, allowing it to be administered at efficacious doses without inducing systemic toxicity. This advantage not only broadens the potential patient population, but also supports its use in combination regimens with minimal compounding toxicity.
Preclinical Efficacy Across Tumor Models
AMG 410 has demonstrated robust antitumor activity in preclinical studies, particularly in solid tumors harboring KRAS mutations. In mouse xenograft models of colorectal cancer, pancreatic ductal adenocarcinoma (PDAC), and non-small cell lung cancer (NSCLC), AMG 410 effectively inhibited ERK phosphorylation, leading to downstream suppression of the MAPK signaling cascade and marked tumor regression.
One of AMG 410’s most promising features is its durable inhibition profile, enabled by its state-independent targeting. This allows continuous suppression of KRAS signaling across dynamic cellular environments where GTP and GDP cycling occurs rapidly. Tumor volume reductions were not only significant but also sustained, even after cessation of dosing in some models, highlighting the compound’s potential for long-term disease control.
In combination studies, AMG 410 showed enhanced therapeutic efficacy when paired with targeted therapies (e.g., MEK or SHP2 inhibitors) and immunotherapies such as PD-1/PD-L1 checkpoint blockers. These combinations led to deeper and more durable responses, suggesting a synergistic effect likely due to pathway co-inhibition and increased immune activation.
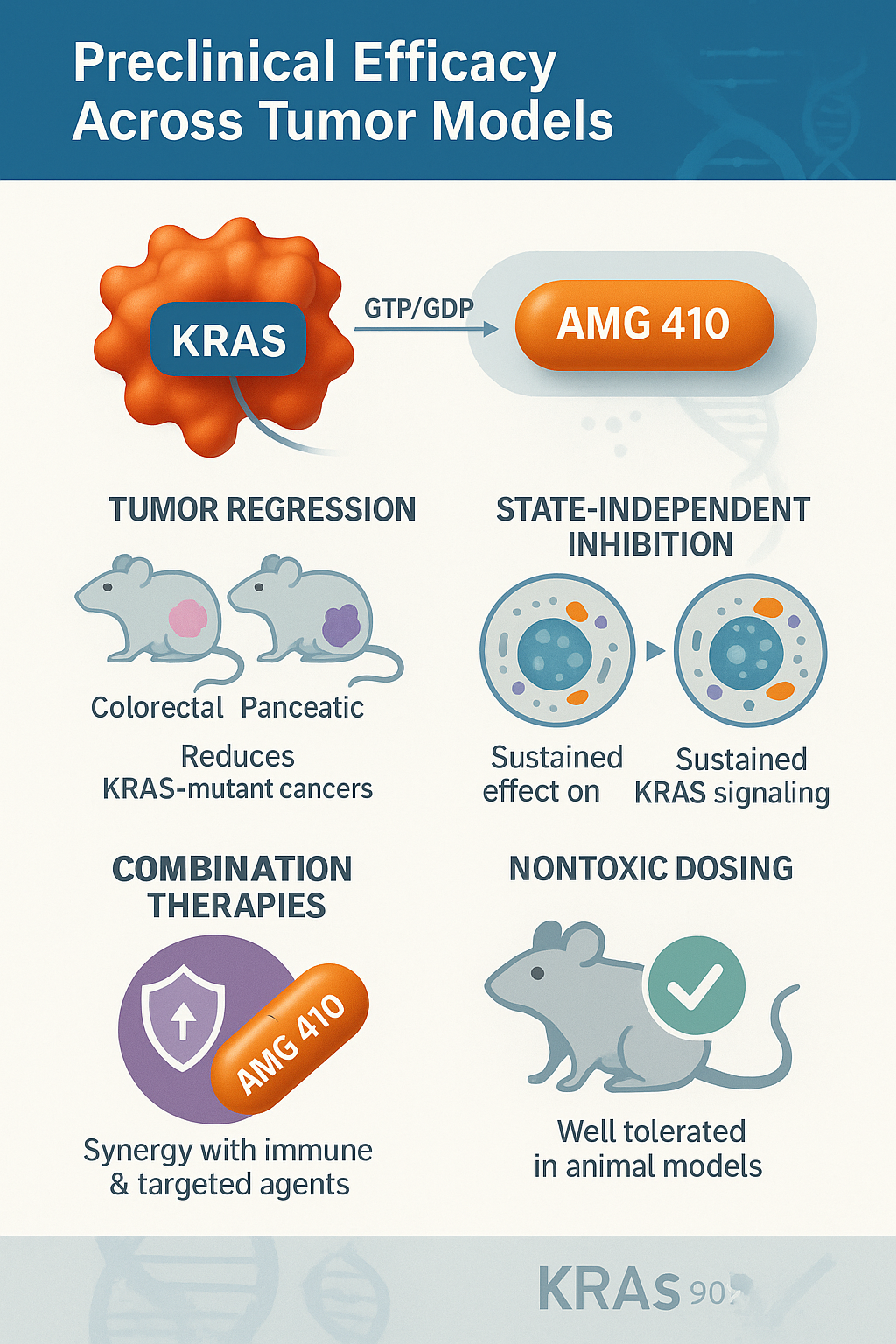
Additionally, toxicology assessments in murine and non-human primate models revealed no dose-limiting toxicities at effective concentrations. These findings strongly support the transition of AMG 410 into first-in-human trials, with ongoing preparations targeting a broad set of KRAS-driven solid tumors.
AMG 410 in the Pipeline
With a compelling preclinical profile, AMG 410 is poised to enter first-in-human clinical trials, offering a much-needed therapeutic avenue for patients with KRAS-mutant solid tumors. The drug’s ability to inhibit a wide spectrum of KRAS mutations across both active (GTP-bound) and inactive (GDP-bound) states opens the door for broader clinical applicability, beyond the limited subset of patients eligible for G12C inhibitors.
According to early development reports, AMG 410 is being positioned for phase I dose-escalation studies, focusing on patients with advanced or metastatic colorectal cancer, pancreatic cancer, and non-small cell lung cancer (NSCLC). These cancers are known for their poor prognosis and limited treatment options, especially when harboring aggressive KRAS mutations like G12D or G13D.
Clinical protocols are expected to include biomarker-guided patient selection, leveraging genetic profiling to ensure optimal responsiveness. In addition, combination arms are likely to be integrated early in clinical trials, building on preclinical synergy observed with MEK inhibitors and immune checkpoint blockers.
Another key point in AMG 410’s development is its favorable safety profile, supported by preclinical toxicology data. This allows investigators to explore higher biologically effective doses with manageable side effects—critical for combination therapy strategies.
If early clinical data replicate the robust preclinical findings, AMG 410 could emerge as a first-in-class broad-spectrum KRAS inhibitor, drastically expanding the treatment landscape for RAS-driven malignancies and complementing the existing class of mutation-specific inhibitors.
Future Outlook
The development of AMG 410 signals a transformative moment in the ongoing battle against KRAS-driven cancers. Unlike earlier inhibitors restricted to a single mutation or nucleotide state, AMG 410’s broad-spectrum, state-independent inhibition offers a versatile platform that could serve as a foundation for next-generation combination therapies.
As KRAS continues to be implicated in cancer resistance and progression, the demand for mutation-agnostic inhibitors is growing. AMG 410 may not only fill this gap but also evolve into a backbone agent in regimens involving MEK inhibitors, SHP2 blockers, and immunotherapies. The ability to selectively suppress KRAS without disrupting HRAS or NRAS functions further elevates AMG 410’s therapeutic value and safety profile.
Moreover, as clinical trials integrate real-time genomic profiling and biomarker-driven stratification, AMG 410 stands to benefit from precision oncology frameworks that align patients with the most appropriate targeted therapies. This approach may accelerate approval timelines and enhance patient outcomes.
In the long term, AMG 410 could be applied not only in first-line therapy but also in overcoming resistance to existing KRAS inhibitors, particularly in tumors harboring compound or adaptive mutations. The flexibility of its non-covalent design makes it an ideal candidate for addressing evolving tumor profiles.
With its broad mutation coverage, strong preclinical efficacy, and combination potential, AMG 410 represents a bold leap toward conquering the KRAS frontier in cancer therapy.
Conclusion
The emergence of AMG 410 marks a pivotal step forward in the evolving landscape of targeted cancer therapy. By offering non-covalent, state-independent inhibition across a broad range of KRAS mutations, AMG 410 addresses many of the limitations faced by earlier generation KRAS inhibitors. Its unique ability to bind both the active (GTP-bound) and inactive (GDP-bound) states of KRAS not only ensures more comprehensive suppression of oncogenic signaling but also opens the door to treating a wider population of patients with KRAS-driven tumors.
Equally important is its exceptional selectivity for KRAS over other RAS isoforms, which supports a favorable safety profile and reduces off-target toxicities. In preclinical studies, AMG 410 has demonstrated potent efficacy in models of colorectal, pancreatic, and lung cancers, with enhanced effects observed when used in combination with targeted or immune-based therapies.
As AMG 410 advances toward clinical development, it holds the potential to become a first-in-class therapy for patients with historically intractable RAS-driven cancers. Its promise lies not just in what it inhibits, but in how it redefines the boundaries of what’s possible in precision oncology.
References
Ryan MB, Corcoran RB. Therapeutic strategies to target RAS-mutant cancers. Nat Rev Clin Oncol. 2018 Nov;15(11):709-720. doi: 10.1038/s41571-018-0105-0. PMID: 30275515.
https://www.nature.com/articles/s41571-018-0105-0
Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, Lanman BA, Werner J, Rapaport AS, San Miguel T, Ortiz R, Osgood T, Sun JR, Zhu X, McCarter JD, Volak LP, Houk BE, Fakih MG, O’Neil BH, Price TJ, Falchook GS, Desai J, Kuo J, Govindan R, Hong DS, Ouyang W, Henary H, Arvedson T, Cee VJ, Lipford JR. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019 Nov;575(7781):217-223. doi: 10.1038/s41586-019-1694-1. Epub 2019 Oct 30. PMID: 31666701.
https://www.nature.com/articles/s41586-019-1694-1
Soula M, Unlu G, Welch R, Chudnovskiy A, Uygur B, Shah V, Alwaseem H, Bunk P, Subramanyam V, Yeh HW, Khan A, Heissel S, Goodarzi H, Victora GD, Beyaz S, Birsoy K. Glycosphingolipid synthesis mediates immune evasion in KRAS-driven cancer. Nature. 2024 Sep;633(8029):451-458. doi: 10.1038/s41586-024-07787-1. Epub 2024 Aug 7. PMID: 39112706; PMCID: PMC11892728.
https://www.nature.com/articles/s41586-024-07787-1
Zhang Y, Ma JA, Zhang HX, Jiang YN, Luo WH. Cancer vaccines: Targeting KRAS-driven cancers. Expert Rev Vaccines. 2020 Feb;19(2):163-173. doi: 10.1080/14760584.2020.1733420. Epub 2020 Mar 14. PMID: 32174221.
https://www.tandfonline.com/doi/abs/10.1080/14760584.2020.1733420