Advancing ADC Therapeutics: Optimizing Combinations for Enhanced Anticancer Efficacy
Abstract
ADCs have become a popular class of drugs for treating hematologic malignancies and solid tumors, undergoing extensive preclinical and clinical research. However, similar to many cytotoxic drugs, the duration of objective responses or clinical benefits produced as a single therapy by ADCs is still limited due to the emergence of resistance mechanisms. Therefore, combining ADCs with other anticancer drugs has become an important direction in the development of ADC therapeutics.
Introduction
Currently, regulatory authorities have approved combinations of ADCs with chemotherapy/chemical immunotherapy for blood tumors. The FDA has also granted breakthrough therapy designation for enfortumab vedotin and pembrolizumab. The most attractive drugs in combination with ADCs are those that have additive or synergistic effects on tumor cells or their microenvironment without unacceptable overlapping toxicities. Combinations of anti-angiogenic drugs, HER2-targeted drugs, DNA damage response agents, and immune checkpoint inhibitors (ICIs) are actively being researched at present.
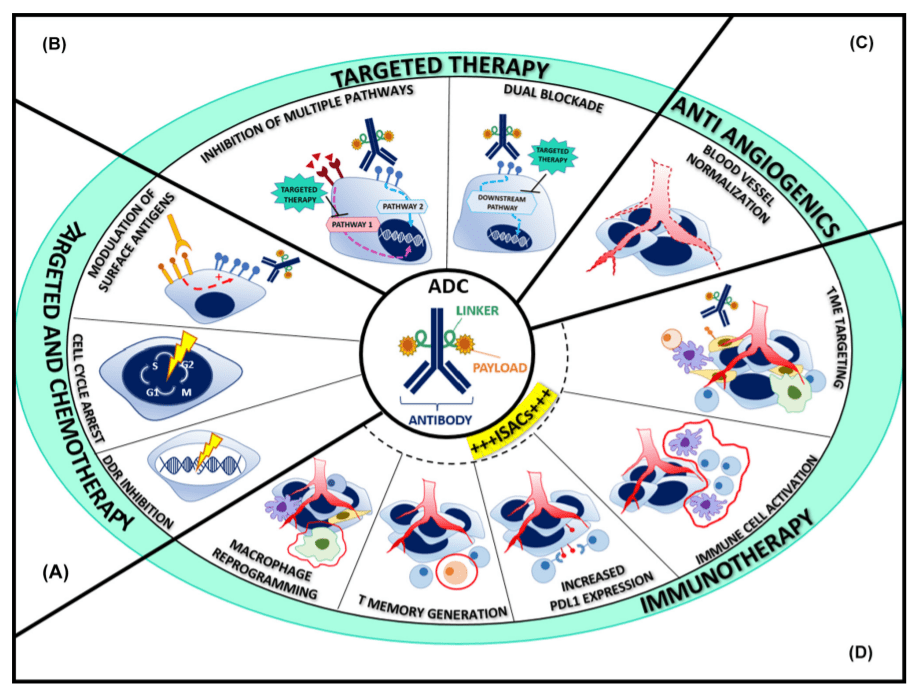
Fig 1. The rationale for antibody–drug conjugate (ADC)-based combination therapies [1].
ADC combined chemotherapy
Translation: The optimal combination of ADCs and chemotherapy drugs requires a better understanding of the unique interactions within the cell cycle and the regulatory effects of cytotoxic companions on surface antigen expression. So far, increasing amounts of preclinical and clinical data have shown promising prospects, providing valuable insights for guiding further drug development.
Cell cycle interactions
DNA-damaging agents that act on the S phase and induce G2/M phase arrest (such as antimetabolites, platinum drugs, and topoisomerase inhibitors) can be combined with microtubule inhibitors. The successful combination of carboplatin with mirvetuximab soravtansine, anetumab ravtanine, or luveltamab tazevibulin in ovarian cancer models illustrates this concept. In early clinical trials, ADCs based on ravtansine, combined with carboplatin or doxorubicin, showed significant efficacy in platinum-sensitive and resistant ovarian cancer patients. Similarly, ADCs based on deruxtecan, combined with capecitabine or cisplatin, demonstrated significant therapeutic effects in gastric and lung cancer patients.
Design of Administration Timing
The administration timing may be related to the design of drug combinations. Microtubule protein aggregation is a crucial component of the ADC internalization mechanism, and the G2/M phase arrest mediated by DNA damage may require some time for microtubule-disrupting agents to sensitize. Studies in colorectal cancer, lung cancer, and breast cancer models have well demonstrated this, showing that continuous administration of SGN-15 (Lewis Y antigen-doxorubicin) and paclitaxel causes more DNA fragmentation compared to simultaneous administration. This observation suggests that adjusting the administration timing, especially delaying the administration of DNA-damaging agents after anti-microtubule drugs, may enhance therapeutic efficacy.
Regulation of Surface Antigens
Chemotherapeutic drugs can regulate the expression of surface antigens targeted by ADCs. In this context, gemcitabine has been demonstrated to upregulate the expression of HER2 in pancreatic cancer cells, and the combination of gemcitabine and trastuzumab emtansine exhibits enhanced efficacy. Interwoven with the aforementioned cell cycle interactions, HER2 upregulation particularly occurs in the G2/M phase, as a result of gemcitabine-mediated inhibition of DNA synthesis.
Translate: Overlapping Toxicity
ADC is essentially chemotherapy, so the improvement in the efficacy of combination regimens is often hindered by unacceptable toxicity. The main toxicities are driven by the metabolism of cytotoxic payload and must be carefully considered when designing combination strategies. These toxicities include peripheral neuropathy caused by MMAE and DM1 derivatives, ocular toxicity caused by MMAF and DM4, gastrointestinal effects of DM1 or topoisomerase inhibitors, liver toxicity caused by calicheamicin derivatives, and almost universal neutropenia and thrombocytopenia.
ADC combined with targeted therapy
Compared to standard chemotherapy, ADC shows an improved therapeutic index and enhanced activity against selectively targeted tumor populations. This makes it an ideal partner for targeted therapy. Various combination strategies can be envisioned to overcome treatment resistance and clonal heterogeneity, triggering stronger inhibition of cancer gene-dependent signaling pathways, increasing the availability of surface antigens, making low antigen-expressing tumors sensitive, and modulating the tumor microenvironment.
Targeting ADC Resistance
Increasing evidence supports that targeted drugs can simultaneously target known ADC resistance mechanisms. For instance, in HER2-resistant patients, the combination of CDK4/6 inhibitors with trastuzumab emtansine has been used because the malignant transformation of breast epithelial cells dependent on HER2 relies on the cell cycle protein D1. Additionally, another critical cell cycle regulatory factor, PLK1, has recently been identified as an upregulated target in acquired and primary trastuzumab emtansine-resistant models. Its inhibitor, volasertib, has been shown to resensitize trastuzumab emtansine both in vitro and in vivo. On the other hand, ADCs may also be an effective combination to modulate resistance mechanisms to targeted drugs. For example, the combination therapy of osimertinib and trastuzumab emtansine results in additional anti-tumor effects, with trastuzumab emtansine able to delay or overcome resistance to osimertinib in EGFR mutant non-small cell lung cancer models.
Anti-angiogenesis
Anti-angiogenic agents can promote ADC penetration and expose tumor cells. The combination of anetumab ravtansine or mirvetuximab soravansine with bevacizumab demonstrates efficacy in preclinical models of ovarian cancer, resulting in a complete response. In a recent phase 1b study, the combination of mirvetuximab soravtansine and bevacizumab was used in heavily pretreated, platinum-resistant, FRα-high ovarian cancer patients, with an ORR (Overall Response Rate) of 39%, surpassing the baseline of the crucial AURELIA trial (27%).
Tyrosine kinase inhibitors (TKIs)
The dual-target blockade by adding TKIs may provide greater selectivity and potentially improve the therapeutic index. In the TEAL study, the combination therapy of trastuzumab emtansine, pan-HER2 inhibitor lapatinib, and albumin-bound paclitaxel showed improved responses in neoadjuvant treatment for HER2+ breast cancer patients compared to standard paclitaxel, trastuzumab, and pertuzumab combination therapy. The combination of trastuzumab emtansine and tucatinib (a more selective anti-HER2 TKI) achieved a 47% objective response rate (ORR) in advanced patients progressing after previous taxane and trastuzumab treatment, including a 36% brain-specific response rate in patients with brain metastases. The use of new-generation ADCs and TKIs may lead to better outcomes.
DNA Damage Response Agents
The development of synthetic lethality by combining drugs targeting the DNA damage response (DDR) with ADC carrying DNA-damaging agents may be a promising strategy for treating genomically unstable tumors. Traditionally, the combination of DDR drugs with chemotherapy has been hindered by intolerable toxicity. The superior activity and tolerance of the new generation of ADCs carrying topoisomerase I inhibitors as payloads make them more suitable as partners. Several clinical trials are exploring this strategy, including combinations such as niraparib and trastuzumab duocarmazine, talazoparib and sacituzumab govitecan, as well as olaparib and trastuzumab deruxtecan.
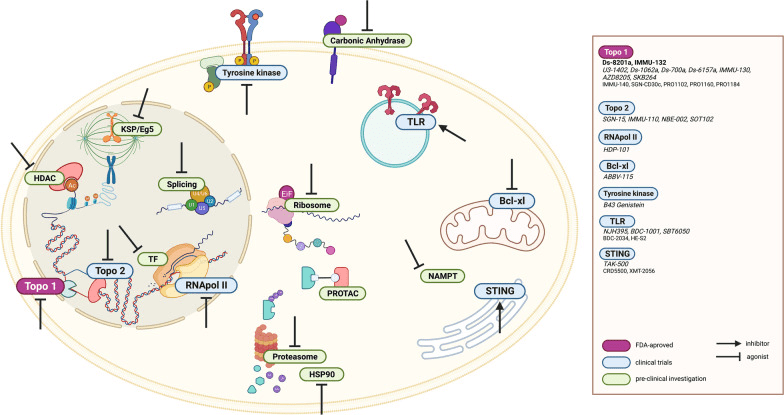
Fig 2. Schematic representation of the ADC payload’s target landscape beyond microtubules and DNA-intercalating agents. Notations: FDA-approved ADCs, ADCs in clinical trials [3].
ADC Combined Immunotherapy
The combination strategy of immunotherapy and ADC has recently entered clinical trials. Although preclinical data and early results from clinical studies indicate enhanced anti-tumor activity, randomized clinical trial results supporting the superiority of this approach over standard treatment are still pending.
Anti-PD-1/PD-L1 and anti-CTLA-4 antibodies
Increasing evidence suggests that ADCs may enhance the efficacy of immunotherapeutic agents. The involved mechanisms are diverse, including the augmentation of immune memory and the expression of immune-regulatory proteins such as PD-L1 and MHC. Some ADCs demonstrate greater potency in preclinical models with intact immune systems, supporting the correlation with their immune-modulating functions.
Various HER2-targeted antibody-drug conjugates (ADCs), including trastuzumab emtansine, trastuzumab deruxtecan, and disitamab vedotin, have been jointly tested with immune checkpoint inhibitors (ICIs) in vitro and in vivo, confirming synergistic activity associated with enhanced homing and activation of immune effector mechanisms. The KATE2 study is the only publicly disclosed randomized trial evaluating the combination of ADCs with ICIs. This study compared the efficacy of trastuzumab emtansine combined with atezolizumab versus trastuzumab emtansine combined with placebo in pre-treated HER2+ breast cancer patients. The combination therapy failed to improve progression-free survival (8.2 vs. 6.2 months, P=0.33), suggesting that the addition of ICIs to HER2-targeted treatment may only be beneficial for the PD-L1 positive population. Despite the disappointing results observed in this randomized trial, clinical exploration in various tumors is still ongoing.
Furthermore, increasing clinical pre-evidence suggests that combination therapy may restore immunosensitivity. For example, in ICI-resistant melanoma and non-small cell lung cancer (NSCLC) patient models, the AXL-specific ADC enapotamab vedotin was tested in combination with anti-PD-1 antibodies. In this combination, the ADC induced T-cell infiltration, enhanced antigen presentation, boosted ICI activity and resulted in pro-inflammatory changes in the tumor microenvironment (TME).
Summary
As a monotherapy, ADCs have proven to possess anti-tumor efficacy and have received approvals in various solid tumors and hematological malignancies. Currently, both the academic and industrial sectors are making extensive efforts to develop the next generation of ADCs by identifying new targets and enhancing their pharmacological effects, as well as exploring combination therapies based on existing ADCs.
However, up to now, the success of combination approaches using first-generation and second-generation ADCs has been limited. This may be attributed to several factors, such as the non-specific expression of targets leading to adverse reactions in normal tissues, overlapping toxicities, insufficient efficacy in different tumor clones, and the emergence of new mechanisms of drug resistance. Therefore, a profound understanding of the pharmacology of ADCs and relevant predictive biomarker combinations is necessary. Preclinical evaluations in well-characterized patient-derived xenograft models will help in selecting the most promising ADC-based combinations. It is believed that the future holds promising prospects for combination therapies based on ADCs.
Reference
- Fuentes-Antrás, J., Genta, S., Vijenthira, A., & Siu, L. L. (2023). Antibody-drug conjugates: in search of partners of choice. Trends in cancer, 9(4), 339–354.
- Tarantino, P., Carmagnani Pestana, R., Corti, C., Modi, S., Bardia, A., Tolaney, S. M., Cortes, J., Soria, J. C., & Curigliano, G. (2022). Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA: a cancer journal for clinicians, 72(2), 165–182.
- Conilh, L., Sadilkova, L., Viricel, W., & Dumontet, C. (2023). Payload diversification: a key step in the development of antibody-drug conjugates. Journal of hematology & oncology, 16(1), 3.
- Tsuchikama, K., Anami, Y., Ha, S. Y. Y., & Yamazaki, C. M. (2024). Exploring the next generation of antibody-drug conjugates. Nature reviews. Clinical oncology, 21(3), 203–223.