| Reference | 1. Clin Cancer Res. 2017 Jan 1;23(1):225-238. doi: 10.1158/1078-0432.CCR-16-0230.
Epub 2016 Jul 20. <br />
The Novel Pan-PIM Kinase Inhibitor, PIM447, Displays Dual Antimyeloma and
Bone-Protective Effects, and Potently Synergizes with Current Standards of Care. <br />
Paíno T(1)(2), Garcia-Gomez A(1)(2), González-Méndez L(1)(2), San-Segundo
L(1)(2), Hernández-García S(1)(2), López-Iglesias AA(1)(2), Algarín EM(1)(2),
Martín-Sánchez M(1)(2), Corbacho D(3), Ortiz-de-Solorzano C(3), Corchete
LA(1)(2), Gutiérrez NC(1)(2), Maetos MV(1)(2), Garayoa M(1)(2), Ocio EM(4)(2). <br />
Author information: <br />
(1)Centro de Investigación del Cáncer-IBMCC (CSIC-Universidad de Salamanca),
Salamanca, Spain.
(2)Complejo Asistencial Universitario de Salamanca-IBSAL, Salamanca, Spain.
(3)Program in Solid Tumors and Biomarkers, Centro de Investigación Médica
Aplicada (CIMA), Universidad de Navarra; Navarra/’s Health Research Institute
(IDISNA), Pamplona, Spain.
(4)Centro de Investigación del Cáncer-IBMCC (CSIC-Universidad de Salamanca),
Salamanca, Spain. [email protected]. <br />
PURPOSE: PIM kinases are a family of serine/threonine kinases recently proposed
as therapeutic targets in oncology. In the present work, we have investigated the
effects of the novel pan-PIM kinase inhibitor, PIM447, on myeloma cells and
myeloma-associated bone disease using different preclinical models.
EXPERIMENTAL DESIGN: In vitro/ex vivo cytotoxicity of PIM447 was evaluated on
myeloma cell lines and patient samples. Synergistic combinations with standard
treatments were analyzed with Calcusyn Software. PIM447 effects on bone cells
were assessed on osteogenic and osteoclastogenic cultures. The mechanisms of
PIM447 were explored by immunoblotting, qPCR, and immunofluorescence. A murine
model of disseminated multiple myeloma was employed for in vivo studies.
RESULTS: PIM447 is cytotoxic for myeloma cells due to cell-cycle disruption and
induction of apoptosis mediated by a decrease in phospho-Bad (Ser112) and c-Myc
levels and the inhibition of mTORC1 pathway. Importantly, PIM447 demonstrates a
very strong synergy with different standard treatments such as bortezomib +
dexamethasone (combination index, CI = 0.002), lenalidomide + dexamethasone (CI =
0.065), and pomalidomide + dexamethasone (CI = 0.077). PIM447 also inhibits in
vitro osteoclast formation and resorption, downregulates key molecules involved
in these processes, and partially disrupts the F-actin ring, while increasing
osteoblast activity and mineralization. Finally, PIM447 significantly reduced the
tumor burden and prevented tumor-associated bone loss in a disseminated murine
model of human myeloma.
CONCLUSIONS: Our results demonstrate dual antitumoral and bone-protective effects
of PIM447. This fact, together with the very strong synergy exhibited with
standard-of-care treatments, supports the future clinical development of this
drug in multiple myeloma. Clin Cancer Res; 23(1); 225-38. ©2016 AACR. <br />
2. Oncotarget. 2016 Sep 27;7(39):63362-63373. doi: 10.18632/oncotarget.11457. <br />
Control of translational activation by PIM kinase in activated B-cell diffuse
large B-cell lymphoma confers sensitivity to inhibition by PIM447. <br />
Peters TL(1), Li L(2), Tula-Sanchez AA(3), Pongtornpipat P(4), Schatz JH(2). <br />
Author information: <br />
(1)Sheila and David Fuente Graduate Program in Cancer Biology, Division of
Hematology-Oncology, Sylvester Comprehensive Cancer Center, University of Miami
Miller School of Medicine, Miami, FL, USA.
(2)Department of Medicine, Division of Hematology-Oncology, Sylvester
Comprehensive Cancer Center, University of Miami Miller School of Medicine,
Miami, FL, USA.
(3)Department of Molecular and Cellular Biology, University of Arizona, Tucson,
AZ, USA.
(4)Bio5 Institute, University of Arizona, Tucson, AZ, USA. <br />
The PIM family kinases promote growth and survival of tumor cells and are
expressed in a wide variety of human cancers. Their potential as therapeutic
targets, however, is complicated by overlapping activities with multiple other
pathways and remains poorly defined in most clinical scenarios. Here we explore
activity of the new pan-PIM inhibitor PIM447 in a variety of lymphoid-derived
tumors. We find strong activity in cell lines derived from the activated B-cell
subtype of diffuse large B-cell lymphoma (ABC-DLBCL). Sensitive lines show lost
activation of the mTORC1 signaling complex and subsequent lost activation of
cap-dependent protein translation. In addition, we characterize recurrent PIM1
protein-coding mutations found in DLBCL clinical samples and find most preserve
the wild-type protein/’s ability to protect cells from apoptosis but do not bypass
activity of PIM447. Pan-PIM inhibition therefore may have an important role to
play in the therapy of selected ABC-DLBCL cases. <br />
3. J Med Chem. 2015 Nov 12;58(21):8373-86. doi: 10.1021/acs.jmedchem.5b01275. Epub
2015 Oct 27. <br />
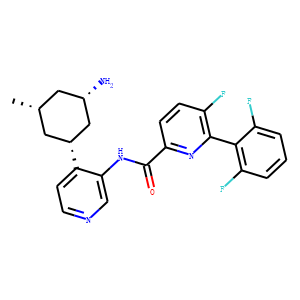
Identification of
N-(4-((1R,3S,5S)-3-Amino-5-methylcyclohexyl)pyridin-3-yl)-6-(2,6-difluorophenyl)-
5-fluoropicolinamide (PIM447), a Potent and Selective Proviral Insertion Site of
Moloney Murine Leukemia (PIM) 1, 2, and 3 Kinase Inhibitor in Clinical Trials for
Hematological Malignancies. <br />
Burger MT, Nishiguchi G, Han W, Lan J, Simmons R, Atallah G, Ding Y, Tamez V,
Zhang Y, Mathur M, Muller K, Bellamacina C, Lindvall MK, Zang R, Huh K, Feucht P,
Zavorotinskaya T, Dai Y, Basham S, Chan J, Ginn E, Aycinena A, Holash J, Castillo
J, Langowski JL, Wang Y, Chen MY, Lambert A, Fritsch C(1), Kauffmann A(1),
Pfister E(1), Vanasse KG(2), Garcia PD. <br />
Author information: <br />
(1)Oncology Research, Novartis Institutes for Biomedical Research , CH-4056,
Basel, Switzerland.
(2)Translational Clinical Oncology, Novartis Institutes for Biomedical Research ,
220 Massachusetts Avenue, Cambridge Massachusetts 02139, United States. <br />
Pan proviral insertion site of Moloney murine leukemia (PIM) 1, 2, and 3 kinase
inhibitors have recently begun to be tested in humans to assess whether pan PIM
kinase inhibition may provide benefit to cancer patients. Herein, the synthesis,
in vitro activity, in vivo activity in an acute myeloid leukemia xenograft model,
and preclinical profile of the potent and selective pan PIM kinase inhibitor
compound 8 (PIM447) are described. Starting from the reported aminopiperidyl pan
PIM kinase inhibitor compound 3, a strategy to improve the microsomal stability
was pursued resulting in the identification of potent aminocyclohexyl pan PIM
inhibitors with high metabolic stability. From this aminocyclohexyl series,
compound 8 entered the clinic in 2012 in multiple myeloma patients and is
currently in several phase 1 trials of cancer patients with hematological
malignancies. <br />
|