BET Inhibitors: A Promising Strategy for Cancer Therapy
ABSTRACT
BET inhibitors are a class of drugs that target proteins called bromodomain and extra-terminal (BET) proteins. These proteins are involved in regulating gene expression, and BET inhibitors work by blocking their ability to bind to chromatin and activate gene transcription.
BET inhibitors have shown promise as potential therapies for cancer, inflammatory diseases, and other conditions that involve dysregulated gene expression. Some examples of BET inhibitors that have been developed and are currently being studied include JQ1, I-BET762, and OTX015.
It is important to note that while BET inhibitors have shown potential in preclinical studies, more research is needed to fully understand their safety and effectiveness in humans. Additionally, as with any new drug, there may be potential side effects and risks associated with their use.
Background information on BET proteins
BET (bromodomain and extraterminal) proteins are a family of transcriptional regulators that play a critical role in gene expression. They are named for their two key domains: the bromodomain, which recognizes and binds to acetylated lysine residues on histone proteins, and the extraterminal (ET) domain, which mediates protein-protein interactions. There are four members of the BET protein family: BRD2, BRD3, BRD4, and BRDT.
BET proteins regulate the expression of a large number of genes involved in a variety of cellular processes, including cell cycle progression, DNA damage response, and apoptosis. The dysregulation of BET proteins has been implicated in various diseases, including cancer, inflammatory disorders, and cardiovascular diseases. In cancer, BET proteins have been shown to play a critical role in tumor development and progression by regulating the expression of oncogenes and other genes involved in cell proliferation and survival. For example, the BET protein BRD4 has been shown to regulate the expression of the MYC oncogene, which is frequently overexpressed in many cancers.
Given their key role in gene expression and disease pathology, BET proteins have emerged as promising targets for therapeutic intervention. Some small-molecule inhibitors of BET proteins have been developed and are currently being evaluated in preclinical and clinical studies for the treatment of various cancers and other diseases.
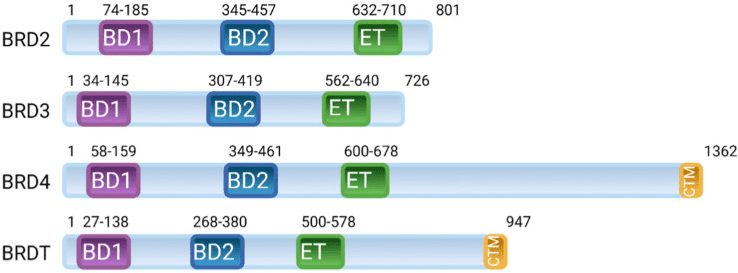
Fig 1 Arrangement of domains in the human bromodomain and extraterminal domain (BET) family of proteins; BRD2, BRD3, BRD4, and BRDT.[1]
Importance of targeting BET proteins in cancer therapy
Targeting BET (bromodomain and extraterminal) proteins has emerged as a promising strategy for the development of new cancer therapies. Dysregulation of BET proteins has been implicated in the development and progression of various cancers, including hematological malignancies and solid tumors. BET proteins play a critical role in the regulation of oncogenic transcriptional programs by binding to acetylated lysine residues on histones, which are key regulators of gene expression.
Inhibition of BET proteins has been shown to have potent anticancer effects by suppressing the expression of oncogenes and inducing apoptosis. BET inhibitors have shown efficacy in preclinical studies, and early-phase clinical trials have shown promising results in patients with hematological malignancies, including multiple myeloma, acute myeloid leukemia, and lymphoma. Importantly, targeting BET proteins has the potential to overcome some of the limitations of current cancer therapies. BET inhibitors have shown activity against cancer cells that are resistant to chemotherapy and targeted therapies, suggesting that they may be effective in combination with other therapies. In addition, BET inhibitors have shown a favorable safety profile in early clinical trials, with few dose-limiting toxicities.
Overall, targeting BET proteins represents a promising approach to the development of new cancer therapies that could improve patient outcomes and overcome treatment resistance. Ongoing preclinical and clinical studies are investigating the potential of BET inhibitors in a range of cancer types, and these studies hold the promise of improving cancer treatment in the future.
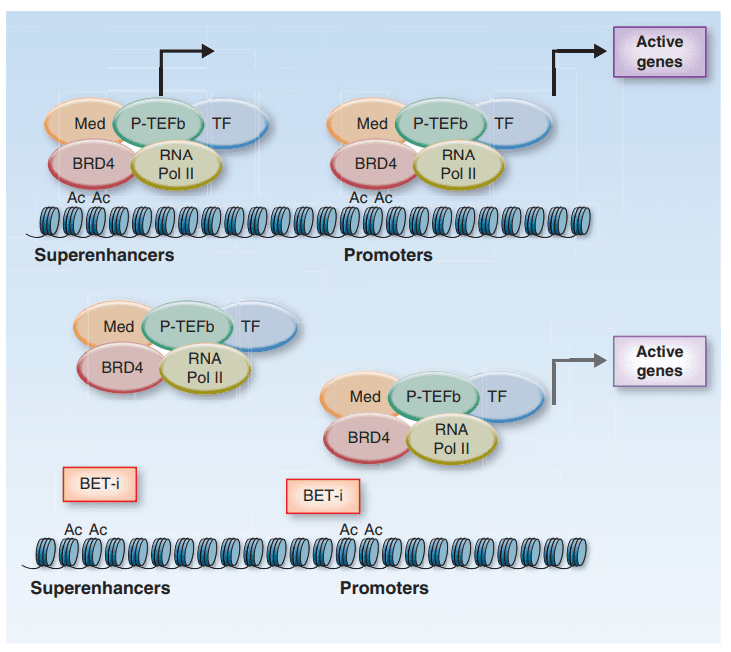
Fig 2. Schematic representation of the mechanism of action of BET inhibitors.[2]
The mechanism of action of BET inhibitors
BET inhibitors are small molecules that have shown potential in cancer therapy. They target bromodomain-containing proteins (BRDs) that are involved in transcriptional regulation. BET inhibitors block the interaction between BRDs and acetylated lysine residues on histones, which disrupts the formation of transcriptional complexes necessary for cancer cell proliferation and survival. The mechanism of action of BET inhibitors involves targeting oncogenic transcription factors such as MYC and disrupting the interaction between BRD4 and MYC. BET inhibitors also target other oncogenic transcription factors and regulators of the cell cycle and DNA damage response pathways. They induce cellular senescence and disrupt protein-protein interactions between BRDs and other proteins involved in cancer signaling pathways.
Identifying predictive biomarkers and overcoming resistance to BET inhibitors are important challenges. Potential biomarkers include MYC expression levels and gene expression signatures associated with BET inhibitor sensitivity. Cancer cells can develop resistance to BET inhibitors through multiple mechanisms. Developing new BET inhibitors and identifying combination therapies that can overcome resistance are important areas of research.
In conclusion, BET inhibitors have shown promising activity in cancer therapy. They disrupt the formation of transcriptional complexes and target oncogenic transcription factors and other regulators. Overcoming resistance and identifying predictive biomarkers will be important for maximizing their therapeutic potential.
Table 1. BET inhibitors.[3]
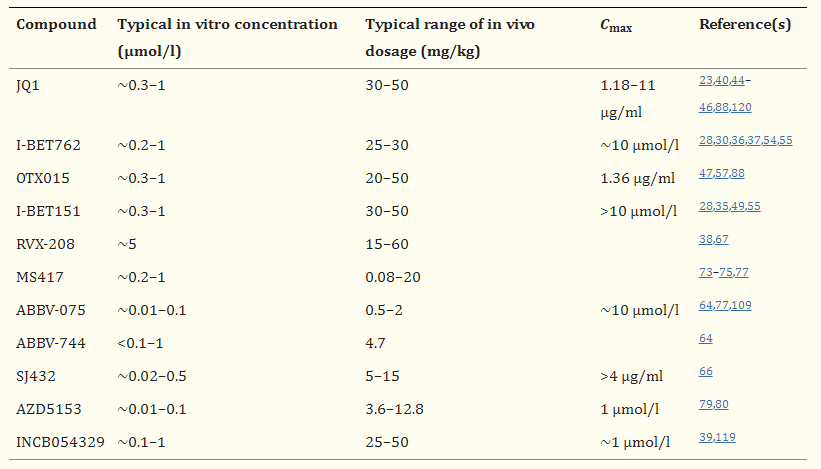
What are the common inhibitors?
BET inhibitors have shown promise in a variety of cancers, including hematologic malignancies, solid tumors, and pediatric cancers. Some of these inhibitors have also been investigated in combination with other therapies, such as chemotherapy and immunotherapy, with promising results. However, further research is needed to determine the optimal dosing and scheduling of these agents, as well as to identify predictive biomarkers of response.
JQ1: JQ1 was the first BET inhibitor to be identified. JQ1 is a small molecule inhibitor that targets the bromodomain and extraterminal (BET) proteins, which are important epigenetic regulators that play a key role in controlling gene expression. JQ1 binds to the bromodomain pocket of BET proteins and blocks their interaction with chromatin, thereby inhibiting the transcriptional activity of genes regulated by BET proteins. One of the key targets of JQ1 is the oncogene MYC, which is frequently overexpressed in various types of cancer. By inhibiting MYC expression, JQ1 can induce cancer cell death and has shown promising results in preclinical studies as a potential therapeutic agent for MYC-driven cancers. However, further research is needed to evaluate the safety and efficacy of JQ1 in clinical trials.
OTX015 (MK-8628): OTX015 is another small molecule inhibitor that targets the bromodomain and extraterminal (BET) proteins, similar to JQ1. OTX015 specifically targets the BET protein BRD2/3/4 and inhibits the interaction between BET proteins and chromatin, thereby inhibiting the transcriptional activity of genes regulated by BET proteins. Like JQ1, one of the key targets of OTX015 is the oncogene MYC, which is frequently overexpressed in various types of cancer. OTX015 has also shown promising results in preclinical studies as a potential therapeutic agent for MYC-driven cancers, and several clinical trials have been conducted to evaluate its safety and efficacy in humans.
I-BET151: I-BET151 is a more potent BET inhibitor than JQ1, with greater selectivity for BRD4. I-BET151 specifically targets the BET protein BRD4 and inhibits the interaction between BRD4 and chromatin, thereby inhibiting the transcriptional activity of genes regulated by BRD4. Like JQ1 and OTX015, I-BET151 has shown promising results in preclinical studies as a potential therapeutic agent for various types of cancer. However, further research is needed to evaluate the safety and efficacy of I-BET151 in clinical trials.
The challenges faced by BET inhibitors
BET (Bromodomain and Extra Terminal) inhibitors have gained significant attention as potential therapeutic targets for cancer treatment. They target the bromodomain proteins, which play a critical role in regulating gene transcription, and thereby have the potential to affect multiple cellular processes involved in tumorigenesis. However, the development of BET inhibitors has faced several challenges that need to be overcome.
One of the challenges is related to the efficacy of BET inhibitors. Although preclinical studies have shown good results, clinical trials have not been successful due to the limited single-agent activity of some inhibitors in solid tumors. In addition, the heterogeneity of cancer poses a challenge to the development of BET inhibitors that can effectively treat various types of cancer. Another challenge is the potential for off-target effects. BET inhibitors may affect the function of other proteins, leading to unintended consequences. Therefore, the development of BET inhibitors that specifically target BET proteins without affecting other proteins is crucial. Furthermore, the potential for drug resistance is another challenge. Tumor cells may develop resistance to BET inhibitors through various mechanisms, including alternative splicing, mutations, and alterations in the expression of BET proteins. Finally, the pharmacokinetic properties of BET inhibitors, such as their solubility, bioavailability, and toxicity, need to be optimized for successful clinical application.
BET degraders are small molecules that are designed to induce the degradation of BET proteins, which are epigenetic regulators of gene expression. BET degraders work by recruiting an E3 ubiquitin ligase to the BET protein, which then marks it for degradation by the cell’s proteasome machinery. As a promising new class of anticancer agents, BET degraders offer several advantages over traditional small molecule inhibitors, including the potential for greater selectivity and improved pharmacokinetics. However, the development of BET degraders is still in the early stages, and there are challenges associated with achieving optimal target engagement and minimizing off-target effects.
At present, several BET degraders have been developed and tested in preclinical studies, with promising results in various types of cancer. However, further research is needed to fully understand the therapeutic potential of BET degraders and to develop effective clinical strategies for their use.
Reference